Hydrolysis of Amphip..
advertisement

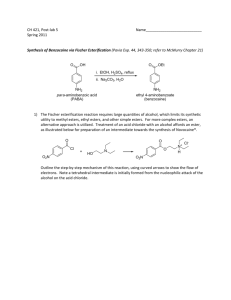
Amphiprotic anions are negative ions that can undergo both acid and base hydrolysis. Here we’ll look at some of these ions and show you how to find which hydrolysis will predominate for a particular ion. It is important to be able to recognize amphiprotic anions. Here is how we do it. HSO3 Formula starts with “H” Firstly, the formulas for amphiprotic anions always start with “H”. It may be just H, or H two. HSO3 Has a negative charge Formula starts with “H” The second thing is amphiprotic anions always have a negative charge. It could be just negative 1 or negative 2. You will find amphiprotic anions on both sides of the acid table. This is because they can undergo both acid hydrolysis and base hydrolysis. The name for an amphiprotic anion is found using the one on the (click) left side of the table. For example, this ion is called the hydrogen oxalate ion. The names of amphiprotic anions (click) start with the word “hydrogen” Or (click) dihydrogen, As in the dihydrogen citrate ion, H2 C6 H5 O7 minus Notice HSO3 minus (click) is called the hydrogen sulphite ion. HSO3 minus is also found on the ion table. It can be called either (click) hydrogen sulphite like it is called on the acid table, Or it can also be called bisulphite These other amphiprotic anions found on the ion table can also be found on both sides of the acid table. You will find it easier to locate them as you use them more. Now that we know how to identify ions as amphiprotic, lets take a closer look at how these can undergo both acid and base hydrolyis. We’ll use the dihydrogen phosphate ion, H2PO4 minus, as an example. Because its on the left side of the acid table, we know it can undergo acid hydrolysis. Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O ( l ) H 3O(aq) 2 HPO4(aq ) acid When H2PO4 minus undergoes acid hydrolysis, it plays the role of an acid Acid Hydrolysis of H2PO4– H 2PO4(aq) acid H 2O ( l ) base And water plays the role of a base H 3O(aq) 2 HPO4(aq ) Acid Hydrolysis of H2PO4– H+ H 2PO4( aq ) acid H 2O ( l ) H 3O(aq) 2 HPO4(aq ) base So a proton is transferred from the H2PO4 minus ion (click) to the water molecule Acid Hydrolysis of H2PO4– H+ H 2PO4(aq) acid H 2O ( l ) H 3O(aq) 2 HPO4(aq ) base When the water gains a proton it forms (click) its conjugate acid, a hydronium ion, H3O plus Acid Hydrolysis of H2PO4– H+ H 2PO4(aq) acid H 2O ( l ) H 3O(aq) 2 HPO4(aq ) base And when H2PO4 minus loses a proton (click) it forms its conjugate base HPO4 2 minus. Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O ( l ) H 3O(aq) 2 HPO4(aq ) So this is the equation for the acid hydrolysis of the dihydrogen phosphate ion, H2PO4 minus. Looking on the acid table, we see that H2PO4 minus (click) is also the right side. This means it can also undergo base hydrolysis Base Hydrolysis of H2PO4– H 2PO4(a q ) H 2O ( l ) OH(aq) H 3PO4(aq) base When H2PO4 minus undergoes base hydrolysis, it plays the role of a base Base Hydrolysis of H2PO4– H 2PO4(aq) H 2O ( l ) base acid And water plays the role of an acid. OH(aq) H 3PO4(aq) Base Hydrolysis of H2PO4– H+ H 2PO4(aq) H 2O ( l ) base OH(aq) H 3PO4(aq) acid So a proton is transferred from a water molecule to the H2PO4 minus ion. Base Hydrolysis of H2PO4– H+ H 2PO4( aq ) H 2O( l ) base OH(aq) H 3PO4(aq) acid When a water molecule loses a proton (click) , it forms its conjugate base, a hydroxide ion, OH minus Base Hydrolysis of H2PO4– H+ H 2PO4( aq ) H 2O( l ) base OH(aq) H 3PO4(aq) acid And when H2PO4 minus gains a proton (click) it forms its conjugate acid H3PO4, phosphoric acid. Base Hydrolysis of H2PO4– H 2PO4( aq ) H 2O( l ) base OH(aq) H 3PO4(aq) acid So this is the equation for the base hydrolysis of H2PO4 minus. In order to find out whether an amphiprotic anion makes a solution acidic or basic, we have to determine which is predominant, acid hydrolysis or base hydrolysis. Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O( l ) H 3O(aq) acid base 2 HPO4(aq ) So we have the acid hydrolysis of dihydrogen phosphate, which produces hydronium ions in solution, Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O( l ) H 3O(aq) acid base 2 HPO4(aq ) Base Hydrolysis of H2PO4– H 2PO4( aq ) H 2O( l ) OH(aq) H 3PO4(aq) base acid And we have the base hydrolysis of dihydrogen phosphate, which produces hydroxide ions in solution. Both of these reactions take place when dihydrogen phosphate is in solution. If: H 3O(aq) OH(aq) then the solution is Acidic OH(aq H O ) 3 (aq ) then the solution is Basic OH(aq then the solution is Neutral H O ) 3 (aq ) Remember that if the hydronium ion concentration in a solution is greater than the hydroxide ion concentration, If: H 3O(aq) OH(aq) Then the solution is acidic then the solution is Acidic If: H 3O(aq) OH(aq) then the solution is Acidic OH(aq H O ) 3 (aq ) then the solution is Basic OH(aq then the solution is Neutral H O ) 3 (aq ) if the hydroxide ion concentration in a solution is greater than the hydronium ion concentration, If: H 3O(aq) OH(aq) then the solution is Acidic OH(aq H O ) 3 (aq ) then the solution is Basic OH(aq then the solution is Neutral H O ) 3 (aq ) Then the solution is basic If: H 3O(aq) OH(aq) then the solution is Acidic OH(aq H O ) 3 (aq ) then the solution is Basic OH(aq then the solution is Neutral H O ) 3 (aq ) And if the hydroxide ion concentration is equal to the hydronium ion concentration, If: H 3O(aq) OH(aq) then the solution is Acidic OH(aq H O ) 3 (aq ) then the solution is Basic OH(aq then the solution is Neutral H O ) 3 (aq ) Then the solution is neutral. H 3O(aq) OH(aq) then the solution is Acidic OH an then the solution is Basic H O If the acid hydrolysis of (aq ) 3 (aq ) amphiprotic anion is predominant then more OH is H than O then the solution is Neutral hydronium produced (aq ) 3 (aq ) hydroxide So if the acid hydrolysis of an amphiprotic anion occurs to a greater extent, or is predominant over base hydrolysis, (click) more hydronium is formed than hydroxide. H 3O(aq) OH(aq) and the solution is Acidic OH an then the solution is Basic H O If the acid hydrolysis of (aq ) 3 (aq ) amphiprotic anion is predominant then more OH is H than O then the solution is Neutral hydronium produced (aq ) 3 (aq ) hydroxide And the solution will be acidic. H 3O(aq) OH(aq) and the solution is Acidic OH(aq H O ) 3 (aq ) then the solution is Basic the base hydrolysis of anthe solution is Neutral OHIf(aq H O then ) 3 is (aqpredominant ) amphiprotic anion then more hydroxide is produced than hydronium If the base hydrolysis of an amphiprotic anion is predominant, then (click) more hydroxide is formed than hydronium H 3O(aq) OH(aq) and the solution is Acidic OH(aq H O ) 3 (aq ) and the solution is Basic the base hydrolysis of anthe solution is Neutral OHIf(aq H O then ) 3 is (aqpredominant ) amphiprotic anion then more hydroxide is produced than hydronium And the solution will be basic. H 2PO4(a q) H 2O( l ) 2 H 3O(aq) HPO4(a q) 2 H 3O(aq) HPO4(aq) ? K a H PO 2 4 H 2PO4(aq) Acid Hydrolysis of H2PO4– Value The extent of acid hydrolysis is determined by (click) the value of the Ka for dihydrogen phosphate. The Ka expression is shown here. H 2PO4(a q) H 2O( l ) 2 H 3O(aq) HPO4(a q) Acid Hydrolysis of H2PO4– 2 H 3O(aq) HPO4(aq) ? K a H PO 2 4 H 2PO4(aq) The concentration of hydronium is in the numerator, so the greater the Ka value the more hydronium ion there is. H 2PO4(a q) H 2O( l ) 2 H 3O(aq) HPO4(a q) Acid Hydrolysis of H2PO4– 2 H 3O(aq) HPO4(aq) ? K a H PO 2 4 H 2PO4(aq) H 2PO4( aq ) H 2O( l ) OH(aq) H 3PO4(aq) BaseofHydrolysis H PO – OH(aq) H 3PO4(aq) K b H PO ? 2 4 H 2PO4(aq) 2 4 Value The extent of base hydrolysis is determined by (click) the value of the Kb for dihydrogen phosphate. The Kb expression is shown here. H 2PO4(a q) H 2O( l ) 2 H 3O(aq) HPO4(a q) Acid Hydrolysis of H2PO4– 2 H 3O(aq) HPO4(aq) ? K a H PO 2 4 H 2PO4(aq) H 2PO4( aq ) H 2O( l ) OH(aq) H 3PO4(aq) BaseofHydrolysis H PO – 2 4 OH(aq) H 3PO4(aq) K b H PO ? 2 4 H 2PO4(aq) The concentration of hydroxide is in the numerator, so the greater the Kb value the more hydroxide ion there is. If: Ka H PO K b H PO then acid hydrolysis predominates If: Kb 2 4 H 2PO4 2 Ka 4 H 2PO4 then base hydrolysis predominates We can summarize by saying, if the Ka for an amphiprotic anion is greater than the Kb for the same ion, If: Ka H PO K b H PO then acid hydrolysis predominates If: Kb 2 4 H 2PO4 2 Ka 4 H 2PO4 then base hydrolysis predominates Then acid hydrolysis will predominate If: Ka H PO K b H PO then acid hydrolysis predominates If: Kb 2 4 2 4 H3O(aq) OH(aq) K then base hydrol y s is predomina tes a H PO H PO 2 4 2 4 And the concentration of H3O plus will be greater than the concentration of OH minus. If: Ka H PO K b H PO then acid hydrolysis predominates If: Kb 2 4 H 2PO4 2 Ka 4 H 2PO4 then base hydrolysis predominates Now, if Kb for an amphiprotic anion is greater than the Ka for the same ion, If: Ka H PO K b H PO then acid hydrolysis predominates If: Kb 2 4 H 2PO4 2 Ka 4 H 2PO4 then base hydrolysis predominates Then base hydrolysis will predominate If: Ka H PO K b H PO then acid hydrolysis predominates If: Kb 2 4 H 2PO4 2 Ka 4 H 2PO4 then base hydrolysis predominates OH(aq H O ) 3 (aq ) And the concentration of OH minus will be greater than the concentration of H3O plus. If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO K a H PO then base hydrolysis predominates 2 4 2 4 Its important to remember these two statements. If Ka is larger than Kb, then acid hydrolysis predominates and if Kb is larger than Ka, then base hydrolysis predominates If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO K a H PO then base hydrolysis predominates 2 4 2 4 The value of Ka for dihydrogen phosphate is found by (click) looking it up on the LEFT side of the table, (click) then reading its value directly from the right side of the row. K a H PO 6.2 10 8 2 4 we’ll make a note of it up here If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO K a H PO then base hydrolysis predominates 2 4 2 4 K a H PO 6.2 10 8 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO K a H PO then base hydrolysis predominates 2 4 2 4 In order to find the value of Kb for H2PO4 minus, we must locate H2PO4 minus (click) up here on the RIGHT side of the table. To find Kb, we must treat this ion as a base, so we look for it on the right side. Ka H PO 6.2 108 If: K a 2 4 H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO K a H PO then base hydrolysis predominates 2 4 2 4 We want to find the value of Kb for this ion. So we want to find the value of Kb for H2PO4 minus. Ka H PO 6.2 108 If: Ka 2 4 H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 The conjugate acid of H2PO4– is H3PO4 The conjugate acid of H2PO4 minus is H3PO4, phosphoric acid Ka H PO 6.2 108 If: Ka 2 4 H PO K b H PO then acid hydrolysis predominates If: K b 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 So the Ka of the conjugate acid of H2PO4 minus is 7.5 × 10–3. Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 Remember, the formula for the Kb of a species such as H2PO4 minus Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 Is Kb equals Kw divided by the Ka of its conjugate acid. The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 Which is Kw divided by the Ka of H3PO4, phosphoric acid. Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 Kw is 1 times 10–14. 2 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 1.0 10 14 12 K b H PO 1.3 1 0 2 4 7.5 10 3 And the Ka of the conjugate acid H3PO4 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 1.0 10 14 12 K b H PO 1.3 1 0 2 4 7.5 10 3 Is 7.5 ×10–3 2 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 So the value for Kb, is 1.0 ×10–14 divided by 7.5 ×10–3 Ka H PO 6.2 108 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b K b H PO 2 4 2 4 2 4 H PO Ka H PO then base hydrolysis predominates 2 4 2 4 Kw K a conjugate acid Kw K a H PO 3 4 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 Which comes out to 1.3 ×10–12 The Ka for the conjugate acid of H2PO4– is 7.5 ×10–3 Ka H PO 6.2 108 K b H PO 1.3 10 12 2 2 If: K a If: K b 4 K b H PO 2 4 H PO K b H PO then acid hydrolysis predominates 4 2 4 4 H PO Ka H PO then base hydrolysis predominates 2 4 Kw K a conjugate acid Kw K a H PO 3 4 1.0 10 14 12 K b H PO 1.3 10 2 4 7.5 10 3 So we’ll make a note of that up here. 2 2 4 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 K b H PO 1.3 10 2 4 We had said that if Ka of H2PO4 minus is greater than its Kb, 12 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 Then acid hydrolysis predominates K b H PO 1.3 10 2 4 12 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 K b H PO 1.3 10 And if Kb of H2PO4 minus is greater than its Ka 2 4 12 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 Then base hydrolysis predominates. K b H PO 1.3 10 2 4 12 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 6.2 × 10–8 is greater than 1.3 × 10–12, > K b H PO 1.3 10 2 4 12 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 > So the Ka of H2PO4 is larger than its Kb K b H PO 1.3 10 2 4 12 If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 > Therefore, acid hydrolysis predominates. K b H PO 1.3 10 2 4 12 K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O ( l ) H 3O(aq) And the equation for the acid hydrolysis of H2PO4 minus is 2 HPO4(aq ) K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 Acid Hydrolysis of H2PO4– H 2PO4(aq) Is H2PO4 minus H 2O ( l ) H 3O(aq) 2 HPO4(aq ) K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 Acid Hydrolysis of H2PO4– H 2PO4(aq) Plus H2O H 2O ( l ) H 3O(aq) 2 HPO4(aq ) K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 Acid Hydrolysis of H2PO4– H 2PO4(aq) Gives H3O plus H 2O ( l ) H 3O(aq) 2 HPO4(aq ) K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 Acid Hydrolysis of H2PO4– H 2PO4(aq) Plus HPO4 2 minus H 2O ( l ) H 3O(aq) 2 HPO4(aq ) K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 Acid Hydrolysis of H2PO4– H2PO4(aq) H2O( l ) Because acid hydrolysis predominates H3O(aq) 2 HPO4(aq ) K a H PO 6.2 10 8 If: K a 2 4 > K b H PO 1.3 10 12 2 4 H PO K b H PO then acid hydrolysis predominates 2 4 2 4 –PO – Acid Hydrolysis of Hof PO Predominant Hydrolysis H 2 42 4 H2PO4(aq) H2O( l ) H3O(aq) 2 HPO4(aq ) We can say that the equation for predominant hydrolysis of H2PO4 minus is (click) this equation, H2PO4 minus acting as an acid in its reaction with water, an forming a hydronium ion. To review, an Amphiprotic anions such as dihydrogen phosphate can act both as and acid (click) and as a base. Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O( l ) H 3O(aq) acid base 2 HPO4(aq ) Base Hydrolysis of H2PO4– H 2PO4( aq ) H 2O( l ) OH(aq) H 3PO4(aq) base acid Undergoing both acid hydrolysis (click) to form a hydronium ion and base hydrolysis to form (click) a hydroxide ion Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O( l ) H 3O(aq) acid base Extent of acid hydrolysis 2 HPO4(aq ) K a H PO 6.2 10 8 2 4 Base Hydrolysis of H2PO4– H 2PO4( aq ) H 2O( l ) OH(aq) H 3PO4(aq) base acid The extent of acid hydrolysis is (click) reflected by the value of its Ka Acid Hydrolysis of H2PO4– H 2PO4(aq) H 2O( l ) H 3O(aq) acid base Extent of acid hydrolysis 2 HPO4(aq ) K a H PO 6.2 10 8 2 4 Base Hydrolysis of H2PO4– H 2PO4( aq ) H 2O( l ) OH(aq) H 3PO4(aq) base acid Extent of base hydrolysis K b H PO 1.3 10 12 2 4 And The extent of base hydrolysis is (click) reflected by the value of its Kb If: K a ion K b ion then acid hydrolysis predominates If: K b ion K a ion then base hydrolysis predominates Once we know the values of Ka and Kb for an amphiprotic anion, we compare them. If (click) Ka for the ion is larger than Kb (click) acid hydrolysis will predominate and more hydronium will be produced than If: K a ion K b ion then acid hydrolysis predominates If: K b ion K a ion then base hydrolysis predominates And if (click) Kb for the ion is larger than Ka (click) base hydrolysis will predominate forming more hydroxide than hydronium. If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 8 > K b H PO 1.3 10 2 4 12 In the example we used, H2PO4 minus, it’s Ka value (click) 6.2 × 10–8 is larger than its Kb value , 1.3 × 10–12, so (click) Acid hydrolysis is predominant. If: K a H PO K b H PO then acid hydrolysis predominates If: K b 2 4 H2PO4 2 Ka 4 H2PO4 then base hydrolysis predominates Ka H PO 6.2 10 2 4 H 2PO4(aq) acid 8 H 2O ( l ) base > K b H PO 1.3 10 2 4 H 3O(aq) 12 2 HPO4(aq ) And the equation for the predominant hydrolysis of dihydrogen phosphate is (click) H2PO4 minus plus water gives H3O plus, plus HPO4 2 minus.