10.5e
advertisement

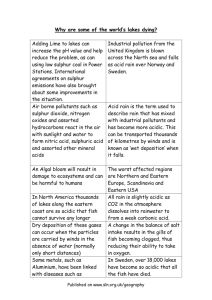
10.5 Acid rain the invisible threat p.29 What is the pH value of rainwater? Why is rainwater acidic? pH value of rainwater is about 5.6 Carbon dioxide + (acidic gas) rainwater rain (with carbonic acid) weak acid Acid rain: pH value is lower than 5.6 Cause of acid rain sulphur dioxide + oxygen + p.29 rainwater (acidic gas) Air pollutants nitrogen oxides (acidic gas) sulphuric acid (strong acid) + rainwater nitric acid (strong acid) Sulphuric acid and nitric acid make pH value of rainwater lower than 5.6 Vehicles、factories and power stations burn coal and crude oil and so acidic gases are formed. - Coal and crude oil contain sulphur. sulphur + oxygen sulphur dioxide When power stations and vehicles burn these fuels, sulphur dioxide will be released. - Under high temperature of burning, nitrogen and oxygen in air will have chemical reaction and nitrogen oxides will be formed. nitrogen + oxygen nitrogen oxides Parthenon (巴特農神殿) is made of marble (大理石). After 24 centuries, it was only a little corroded. The speed of corrosion increased rapidly in the recent 30 years. Some parts of the building collapsed because of acid rain. Some natural phenomena may also form acidic gases. Volcanic explosion releases sulphur dioxide sulphur + oxygen sulphur dioxide Lightning forms nitrogen oxides nitrogen + oxygen nitrogen oxides p.29 What are the effects of acid rain on humans and the environment? Effects of acid rain p.30 Corroding buildings Corroding metals Damaging plants Killing aquatic life Corroding buildings - From the results of Experiment Centre 10.9 and 10.10, we know that acids are corrosive to metals and calcium carbonate. => Acid rain corrodes buildings or statues made of metals or marble. lime - If the rain is more water metal acidic, the speed of corrosion will be higher. dilute hydrochloric acid Damaging our forests - Acid rain: => dissolves some essential nutrients in soil. => affects absorption of required nutrients of the plants. => affects healthy growth of plants and kills them. In the New Territories, some farmers found that leaves of vegetables turn yellow and ‘get burns’ after raining. Killing aquatic life - Acid rain falls into rivers and lakes and makes the water acidic. - When the pH value of water is below 5, most fishes will die. - Besides, acid rain: => dissolves some toxic minerals in soil. => leads the toxic minerals flowing into rivers and lakes. => kills aquatic life and destroys eggs of fishes. Effects of acid rain on humans - Acid rain may cause respiratory diseases and skin allergy. Rainwater with the highest acidity In 1983, pH value of acid rain in Scotland is as low as at 1.87. More acidic than lemon juice! Is the acid rain problem serious? p.33 - Acid rain is not new. - Cities continuously. It wasand firstindustries observeddevelop 100 years ago => Damaging effect acid rain is more when factories wereofconcentrated in obvious. European cities. => Concentration of air pollutants increases - The following map shows ‘Global acid rain pollution’ problem: pH lower than 4.2 pH 4.2 - 4.5 pH 4.6 – 5.0 region with mild acid rain Is there any acid rain problem in Hong Kong? The following questions may help you to find the answer. > What is the main source of air pollutants in Hong Kong? > What is the situation of air pollution in China? > Will air pollutants in China affect Hong Kong? How can we control acid rain? p.33 - To control acid rain, we should take appropriate measures to reduce the formation of acidic gases. What are the methods to reduce the formation of acidic gases? Methods of reducing acidic gas exhaust: 1 Save resources and reduce burning of fossil fuels. 2 Use fuel with lower sulphur content. 3 Use the energy sources without producing acidic gases. e.g. solar power, wind power... 4 Remove the acidic gases after burning. e.g.catalytic converter in cars catalytic converter 5 Educate the public on effects of acid rain and the methods of reducing acid rain. - Acid rain problem in Hong Kong is not a local problem, but a regional problem. - The HKSAR government and the Guangdong Provincial government join together to control acid rain. e.g. Set air pollutant emission reduction targets => Reduce the regional emission of sulphur dioxide by 40% and nitrogen oxides by 20% before 2010.