Acid Rain
advertisement

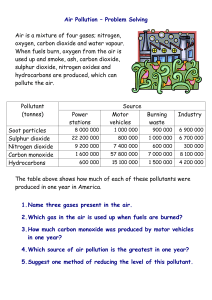
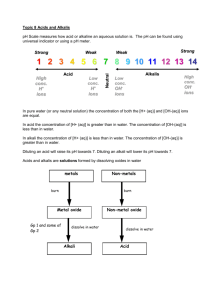
- Rain or precipitation with a pH lower than 5.6 - Normal rain water has a pH lower than 7 - Carbon dioxide dissolves in water to form carbonic acid -Carbonic acid ionizes water to form hydronium ions Oh NO! -Electricity generation, factories and motor vehicles -Burning of fuels -High temperature causes sulphur to react with oxygen to produce sulphur dioxide -High temperature causes nitrogen to react with oxygen to produce nitrogen dioxide. - Gases dissolve in water to produce acids -Volcanic eruption that emits acid-producing gases like sulphur dioxide. -Dimethyl sulphide oxidized by phytoplanktons in oceans to produce sulphur dioxide -Sulphur dioxide dissolves in water to form sulphuric acid - wildfires • Affect the soil -some of the organism do not adapt to such a acidic habitat - the acid rain makes some forests have been growing more and more slowly LIKE THIS! • The aquatic animals - some aquatic organism cannot bear the acidity of the water caused by the acid rain and this greatly affects their living. -Sulphuric acid in polluted precipitation interferes with the fish's proficiency to take in oxygen, salt, and nutrients • The rock and man-made materials -acid rain also damages man-made materials and structures - toxic gases may even be released and this posts a threat to our human’s health • About the sulphur dioxide -use low sulphur-content coal • -Use Scrubbers ~ The sulphur dioxide gas reacts with the lime to produce a solid of calcium sulphate. Photos of scrubber • About the nitrogen oxides - installation of catalytic converters ~This converts nitrogen oxides, carbon dioxides and unburned hydrocarbons into a cleaner state to reduce the oxides of nitrogen.