Hypotonic, Hypertonic, Isotonic Solutions: Cell Biology Notes
advertisement

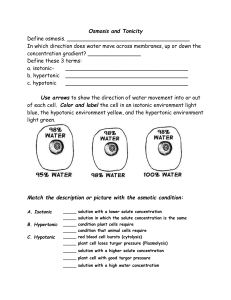
Biological Solutions Notes Objective: You will be able to explain the differences between hypotonic, hypertonic and isotonic solutions and describe their effects on cells. Itinerary: 1. Powerpoint (definitions) 2. Potato experiment demonstration 3. Celery demonstration Word Hypotonic solution Definition a. Greater concentration of solute inside cell than outside Picture Word Hypotonic solution Definition b. Water leaves solution and goes into the cell. c. Cell will get bigger and can burst! Picture Word Definition Hypertonic solution a. Greater concentration of solute outside of cell than inside Picture Word Hypertonic solution Definition b. Water leaves cell and goes into solution. Cell will shrivel up. c. Can lead to dehydration Picture Word Isotonic solution Definition a. Concentration inside cells is equal to that of the solution b. Cell is at equilibrium Picture Which is which? Label each one as: hypotonic, hypertonic or isotonic 1. 2. 3. Which is which? Label each one as: hypotonic, hypertonic or isotonic 1. 2. 3. Type of solution Definition What happens to cells in this environment? Hypotonic Greater concentration of solute inside cell than outside Water goes into the cell. Cell will get bigger and can burst! Hypertonic Greater concentration of solute outside of cell than inside Water leaves the cell. Cell will get shrivel up and dehydrate Isotonic Concentration inside Cell is at cells is equal to that equilibrium of the solution