Science Lab Book
advertisement

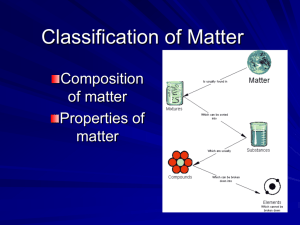
Science Lab Book What are Physical Changes in Matter? Vocabulary Define & illustrate: • Matter – is anything that has mass and takes up space • Solid – particles locked in place, although still vibrating (ice) • Liquid – particles close to each other; move quickly and slide past each other quickly (water) • Gas – particles are far apart and moving quickly • Physical change – is a change that does not result in a new substance • Change of state – occurs when a substance changes from one state to another • Mixture – is two or more substances that are combined without being changed Vocabulary Read pages 148 – 149 Brain Pop: Compounds and Mixtures • Compound – a substance made of two or more kinds of atoms that are chemically combined Examples: Sulfur reacts with oxygen to form sulfur dioxide (substance burns). Iron reacts with oxygen in the presence of water to form rust. Sulfur in the match head is what helps the match light quickly (burning). • Mixture – is two or more substances that are combined without being changed Examples: Trail mix; salads; salt water; fog; air; sand; soil; lemonade; chocolate milk; Vocabulary • Melting point – the temperature at which a solid changes state into a liquid • Boiling point – the temperature at which a liquid changes state into a gas • Freezing point – the temperature a which a liquid changes state into a solid • Evaporation – a liquid slowly turning into a gas • Condensation – a gas turning into a liquid Physical Change Write and illustrate everything you know about a physical change. (Use your textbook as a reference.) Physical Change – is a change that does not result in a new substance Evidence that could be present to indicate a physical change: • No new substance has been created • Change of shape (cutting a piece of paper) • Change of state (going from a solid to a liquid such as ice to water vapor) • Change in size • Mixture (trail mix) • Solutions (salt dissolving into water) • Dissolving Chemical Change • Write and illustrate everything you know about a physical change. (Use your textbook as a reference.) • A chemical change (chemical reaction) is a change that results in one or more new substances. • Reacts means “goes through a chemical change”. Evidence that could be present to indicates a chemical change: New substances are produced with physical properties different form the starting substances • Change in color (bread baking and changing form white to brown) • Smell (rotten eggs) • New physical property (iron rusting) • Substance given off (sulfur burning on a match) • Gas being produced (bubbles) • Light or heat being given off More examples: silver tarnishes, candles burn (both chemical and physical change), milk sours, toast browns, battery acid eats cloth, steel rust, campfire How is a physical change different from a chemical change? Pages to review in your science text book.: • Read page 130-137 “What is Matter Made of?” • Read pages 144-149 “What are Physical Changes in Matter?” • Read Pages 158-163 “What are Chemical Changes in Matter?” Class Work Review: Power Points: • “Chemical Change” • “Name that Change” • “Chemical and Physical Changes?” • “Physical and Chemical Changes A Write on Activity” Physical and Chemical Changes Technology Work • http://www.quia.com/quiz/303980.html (physical & chemical change) - individual work • http://www.harcourtschool.com/activity/hotp late/ (melting and boiling points) - group