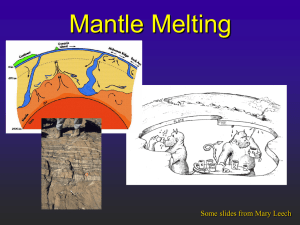
Overview: the geochemist's Earth (reservoirs, budgets and processes)
advertisement

Mantle geochemistry: How geochemists see the deep Earth Don DePaolo/Stan Hart CIDER - KITP Summer School Lecture #1, July 2004 Geochemistry 50 years ago dealt with fewer questions and parameters, e.g. Birch (1952) • How does meteorite chemistry compare with seismic properties of Earth’s interior • Is it Olivine+Pyroxene or other phases ? • How much Fe in the mantle ? • How much Al,Ca,Na,K (“sialic components”) is in the mantle ? • 11 elements of interest: O,Mg,Si,Fe,Ni,Al,Ca,S,Na,K,P What can geochemistry do in 2004? • The earth is made of 90 or so chemical elements, about 30 w/isotopic variations • Chemical/isotopic characteristics can be tied to geological processes - mantle isotopic chemistry is a tracer • We can tell where a particular piece of mantle has been in the past and/or what has happened to it • Radiogenic isotopes provide clocks as well as tracers Questions for geochemistry • How deeply does near surface material circulate into the mantle? On what time scale? • Does the mantle have large scale chemical structure (layering?) • Does the core exchange material with the mantle? (Do plumes come from the CMB?) • What are the characteristics of mantle convection in terms of its ability to stir and homogenize heterogeneous materials? • What features of mantle seismic heterogeneity are thermal and which are chemical? • What aspects of mantle structure are congenital?, of recent origin?; steady-state features? Components of geochemistry • Petrology of the mantle (proportions of minerals or rock types - e.g. lherzolite, harzburgite, eclogite, pyroxenite) • Melting of the mantle • Trace element composition of the mantle (doesn’t affect mineralogy, but can be indicative of history) • Trace element composition II (water and CO2) - affects melting behavior. • Isotopic composition of the mantle (from radioactive decay, input from surface reservoirs, input from core?) • Sampling of the mantle (scale of sampling by magmatism; sampling biases, invisible reservoirs) • Material balance - the sum of the parts must equal the whole Earth for every element and isotope (1) (2) Upper mantle (100) Lower mantle (300) (200) Mass in units of 1025g Oceanic lithosphere ≈ 10 Continental lithosphere ≈ 5 (1) (2) Upper mantle (100) Lower mantle (300) (200) Mass in units of 1025g Consider this: (1) “Heterogeneities” are introduced from the top and the bottom (2) Magmatism samples only the top and the bottom There are key elements of the system where chemistry is done. (Most of what we infer about the mantle depends on how well we understand the processes.) Choose one: There are... (a) too few (b) too many (c) just the right number ...of isotopic tracers There are stable isotopes too ! Why the crustal reservoirs matter... Why the crustal reservoirs matter... Depleted mantle 103 . MORB = 10-14 sec-1 Heterogeneity thickness (km) 102 101 Other lava sequences Laminar shear 100 10-1 Turbulent shear 10-2 UM rocks 10-3 Chemical Diffusion -4 10 0 500 1000 Age (Ma) 1500 2000 Thickness of geochemical anomalies Geochemically Anomalous Layer (Thickness = h Isotopic contrast = ²R h Concentration = Ch Background mantle concentration = Cb Background mantle that must be averaged with anomalous material (effective anomaly thickness): b = h Ch ²R h Cb ²R a Making heterogeneity at a mid-ocean ridge... Mid-ocean ridge factory Hydrothermally altered sediment basalt, gabbro harzburgite strongly depleted lherzolite Incipiently depleted lherzolite H2O-enhanced melting region unmodified lherzolite -20 sediment basalt, gabbro harzburgite strongly depleted lherzolite Incipiently depleted lherzolite unmodified lherzolite 0.1 b.y. later Nd 0 +20 -20 1.0 b.y. later Nd 0 +20 Anything systematic about distribution of heterogeneities? Anything systematic about distribution of heterogeneities? Bulk Earth Younger cont. crust Lower cont. crust Upper cont. crust Older cont. crust Anything systematic about distribution of heterogeneities? Bulk Earth Chondrites Distribution of isotopic ratios among ocean islands is not entirely random Al Hofmann’s analysis, 2003 120 Mid-Atlantic Ridge (data from PETDB) Material balance for Sm-Nd... 100 Average Mantle Count 80 60 40 Bulk Earth 20 0 -8 -4 0 4 8 Nd 12 16 15 “DM” 10 Epsilon Nd An example of heterogeneity on various scales Nd isotopes in MAR basalts. 5 to 10 units of variation can be found over 10km or 10,000km along the ridge. The entire range of values observed worldwide (in all types of oceanic basalts) is found along the ridge 5 AM “PM” 0 -5 Mid-Atlantic Ridge (data from PETDB) -10 -60 -40 -20 0 20 LATITUDE 40 60 80 Recycled MORB Primitive The helium problem Melting region Sampling issues: Pt. 1 edge center 9.0 8.5 Present 8.0 200 Ka 400 Ka Mahukona Kohala Nd 7.0 600 Ka 800 Ka Melt Supply max = 5 cm/yr 6.5 6.0 100 1.0 Mauna Kea HSDP Hualalai 0.1 0.05 0.001 N 300 400 500 600 25 20 3He/4He (R/Ra) Loihi 200 Model Age (ka) 0.5 0.3 Mauna Loa HSDP Mauna Kea (Bryce & DePaolo, 2002) 0.9 0.7 Kilauea Magma Capture Area 7.5 HSDP Mauna Kea (Kurz, 2002) 15 10 MORB Range 5 20 km 0 100 200 300 400 Model Age (ka) 500 600 3 5 Width of melting region 10 4 He/ He 20 25 30 15 35 0 500 He-3 anomaly ( Pb anomaly?) Depth (km) Sr anomaly 1000 1500 2000 Sr 2500 Core or core-mantling dense layer 3000 He 0.7025 87 Sr/ 86 0.7035 Sr 40 Things may get even more interesting when we model the melting in the context of the flow (M. Jull, unfinished, 2003) Melting versus tracers... (Modeling from Jull & Ribe, 2002) Sampling issues, Pt. 2: Over what vertical distance are isotopic ratios averaged? Erupted Lava Storage Chamber (V r) Lithosphere ²z Partial melt zone ( , w) z=0 W z Plume i DePaolo, JGR, 1996 18.40 12 18.45 Pb/204 Pb 14 10 206 8 6 4 200 400 600 800 Depth (meters) 1000 18.50 18.55 18.60 18.65 200 400 600 800 Depth (meters) 1000 104 Hydrodynamic Dispersivity (m) 3 He/4He (R/Ra) Estimating dispersivity in the Hawaii melting region He Pb Sr Nd 103 1 km 102 Mauna Kea (380-1000m) 101 0 From DePaolo, JGR, 1996 5 10 w/W 15 20 For MOR’s the channeling instability may apply; makes for very large vertical dispersion - i.e. lots of averaging. May not be the case for plumes (?) Spiegelman et. al, 2001 (JGR, 106, 2061-2077) OK, so what do we think we know......? Where we are going in the next 2 weeks.... Geochemistry Tutorials....