Chemical Reactions: Equations, Types, and Equilibrium
advertisement
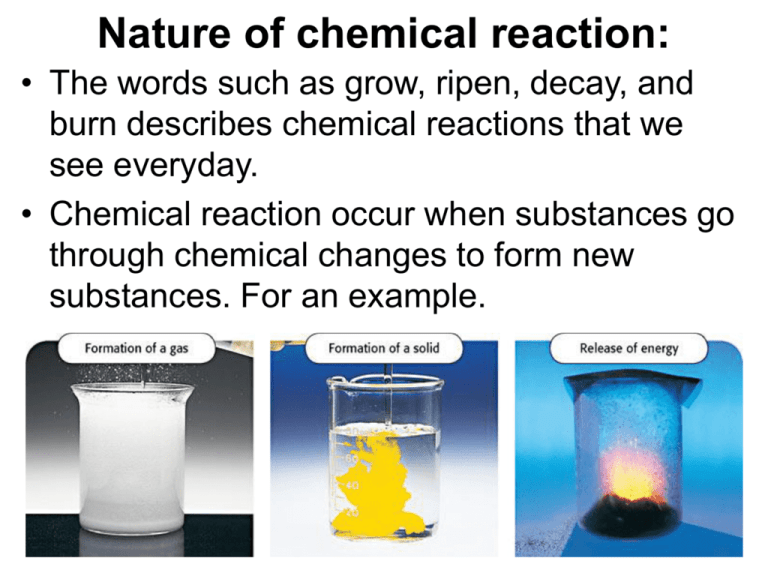
Nature of chemical reaction: • The words such as grow, ripen, decay, and burn describes chemical reactions that we see everyday. • Chemical reaction occur when substances go through chemical changes to form new substances. For an example. Chemical equation: Chem. equation uses symbols to represent a chem. Reaction and shows the relationship between the reactants and products of a reaction. CH4 + 2O2 → CO2 + 2H2O + ENERGY CH4 + 2O2 are called reactants that participate in reaction, and reactants are always written at left side of equation. CO2 + 2H2O called products that is formed as a result of reaction and products are always written at right side of equation. Chemical equation: An arrow(→) is put between reactants and products to indicate that products are produced or yield from reactants. The reactants and products contain same type of atoms. Atoms cannot be created or destroyed during chemical reaction. A balanced equation tells you the mole ratio, or proportion of reactants and products, in a chemical reaction. It means relative amounts of reactants and products. Mole ratio can be converted to masses. Balanced equation and mole ratio: • Law of definite proportions: A compound always contains the same elements in the same proportions regardless of how the compound is made or how much the compound is formed. Energy and chemical reactions: • Chemical reactions are breaking of old bonds from reactant-molecules and formation of new bonds in product-molecules. • Chemical reactions involve changes in energy. Photosynthesis is an endothermic reaction. • Energy is released (exothermic) during formation of bonds and energy is required for breaking the bonds endothermic) • Energy is conserved in chemical reactions. • The energy that is stored in form of chemical bonds is called chemical energy. Explain the types of reactions: 1. Combustion Reaction: A substance such as wood, natural gas, or propane combines with oxygen releasing a large amount of energy and produces carbon dioxide and water. CH4 + 2O2 ----- CO2 + 2H2O + energy Methane Oxygen Carbon dioxide C3H8 + 5 O2 ---- 3 CO2 + 4 H2O + energy Propane 2. Exothermic Reaction: In this type of reaction, generally energy in form of heat is released. For example all combustion reactions are exothermic. “Meals ready to eat” packets and for fixing “Clogged drain” process are also based on exothermic reaction. Chemical energy: reactants vs. products • The hump in the middle of each graph represents the energy required to start the reaction. 3. Endothermic Reaction: In this type of reaction, generally energy in form of heat is absorbed. For example, • “instant cold pack” as a treatment for aching muscles is endothermic reaction. • Photosynthesis is an endothermic reaction in which, reaction occur taking CO2 from air, H2O from soil, green pigment chlorophyll present in plants and light energy from sun is absorbed to form sugar (stored in form of product) and oxygen gas that is released into the air. light energy from sun is absorbed 6CO2 + 6H2O → C6H12O6 + 6O2 Define the following terms: 4. Dissolution Reaction: Dissolution reaction occurs when an ionic compound dissolves in water to make an ionic solution. For example, cold pack reaction is also called dissolution reaction. Insoluble: A term to describe a substance that does not dissolve in water. Precipitates: Solid particles are formed when two ionic compounds are added to each other. Free radical: An atom or a group of atoms that has one unpaired electron. Oxidation-Reduction Reaction: Any chemical change in which one substance is oxidized (loses electrons) and another substance is reduced (gains electrons) This reaction is also called as Redox reaction Explain the steps of balancing chemical equations Example: Al + O2 ---- Al2O3 Step-1: Calculate the number of atoms of each type on both sides of equation. Reactant side Al = 1 Product side Al = 2 O=2 O=3 Atoms are not equal on reactants and product side, therefore equation is unbalanced. Step-2: To balance the equation, choose the correct coefficient and place in front of any reactant and/or product molecule to make the number of atoms equal on both sides. First make Al-atoms equal. 4 Al + O2 --- 2 Al2O3 Now make oxygen atom equal. Step-3: 4 Al + 3 O2 --- 2 Al2O3 Now you can check that Both “Al “ and Oxygen-“O” atoms are equal on both sides of equation. The equation is balanced. Practice: Balance the following reactions. 1) Fe + O2 --- Fe2O3 2) P4 + O2 ---- P2O5 3) KOH + HCl ---- KCl + H2O 4) Ba(NO3) + NaI ---- BaI2 + Na (NO3) 5) C2H8 + O2 ----- CO2 + H2O 6) CaCO3 --- CaO + CO2 7) CuCl2 + Al --- Al Cl3 + Cu 8) H2O2 ----- H2O + O2 9) NH3 ----- N2 + H2 Synthesis Reaction: gen. eq. A + B AB • The reaction in which two or more substances combined to form a new compound. • When many Ethylene molecules combine then polyethylene is produced. It is synthesis or addition reaction. • 2Na + Cl2 --- 2NaCl • sodium metal + chlorine gas table salt. 6. Decomposition Reaction: AB -- A + B • The reaction in which a substance –a bigger molecule is broken apart into smaller substances. • Digestion and cracking oil are decomposition reactions. Examples: • 2H2O --- H2 + O2 2NaHCO3 ----- Na2CO3 + CO2 + H2O Sodium hydrogen carbonate Sodium chloride + carbon dioxide gas + water Single displacement Reaction gen. Eq. AX + B BX + A • The reaction in which one element or radical takes the place of another element or radical in a compound. • In general, a more reactive element will take place of a less reactive one in singledisplacement reaction. • Electroplating of silver or gold is single displacement reaction. • 3CuCl2 + 2Al 2AlCl3 + 3 Cu Copper chloride solution + Aluminum --- Aluminum chloride + Copper Double displacement Reaction: AX + BY AY + BX • The reaction in which a gas, a solid precipitate, or a molecular compound is formed from the apparent exchange of atoms or ions between two compounds. • Pb(NO3)2 + 2 KI PbI2 + 2K(NO3) • Lead nitrate + Potassium iodide -- Lead iodide + Potassium nitrate • In general ions appear to be exchanged between compounds in double displacement reaction. Reaction rate and equilibrium: • Enzymes: A molecule, ether protein or RNA, that acts as catalyst in biochemical reactions. • The reactant in reactions catalyzed by enzymes. For enzymes to catalyze a reaction, the enzyme and substrate must fit like lock and its key. For example, Enzyme Substrate Role of the enzyme Amylase starch To break down starch into smaller molecules Cellulase cellulose To break down long into sugars molecules DNA nucleic acid To build up DNA polymarase chains in cell nuclei Lipase fat To break down fat into smaller molecules Protease protein To break down proteins into amino acids • Catalysts: A substance that changes the rate of reaction without being consumed means does not participate actively in chemical reaction. N2 + 3H2 → 2NH3 Product Ammonia is produced faster in presence of iron catalyst. – Catalysts used in various industries to increase rate of various chemical reaction and also to increase percentage of products in less amount of time. – Economically cost of reactions can be decreased and more profit can be achieved. • Inhibitors: The substances that slow reactions are called inhibitors. • Chemical equilibrium: Some chemical reactions, however go in both directions, which results in an equilibrium system. • Reversible reaction: Reactants changes to product and under same condition, products get decomposed into reacants. “A chemical equilibrium is a state in which a reversible chemical reaction is proceeding in both forward and reverse direction with equal rate at given set of conditions.” Foe example, CaCO3 ↔ CaO + CO2 In this reversible chemical reaction, if pressure, temperature and concentration of any reactant and product does not change then forward and reverse reactions take place at the same rate. The effects of change on equilibrium: • Condition • Temperature Effect Increasing temperature favors the reaction that absorbs energy. • Pressure Increasing pressure favors the reaction that produces fewer molecules of gas. • Concentration Increasing the concentration of one substance favors the reaction that produces less of that substance • Ammonia is a chemical building block used to make products such as fertilizers, dyes, plastics, cosmetics, cleaning products, and fire retardants. The Haber process, which is used to make ammonia industrially, is N2(g) + 3H2(g) → 2NH3(g) + energy • Le Chatelier’s principle: If a change is made to a system in chemical equilibrium, the equilibrium shifts to oppose the change until a new equilibrium is reached automatically by the system. • Chatelier’s principle predicts changes in equilibrium. • Le Chatelier’s principle can be used to control reaction. • The percentage of yield can be increased in reversible reactions using Le Chatelier’s principle.








