Arenes and phenols powerpoint
advertisement
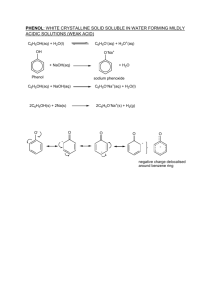
A2 Chemistry Chapter 2 Chapter 2 Objectives Properties of Phenol Delocalisation of Benzene Phenol + NaOH Nitration of Benzene Phenol + Na(s) Halogenation of Benzene Phenol + Br2 SAQ 2.1 SAQ 2.3 SAQ 2.6 A2: Arenes Chapter 2 Objectives (1-8) 1. 2. 3. 4. Show understanding of the concept of delocalisation of electrons as used in a model of benzene Describe electrophilic substitution of arenes with concentrated nitric acid in the presence of concentrated sulphuric acid, a halogen in the presence of a halogen carrier, and a halogenoalkane such as chloromethane in the presence of a halogen carrier (Friedel-Crafts reaction). Describe the mechanism of electrophilic substitution in arenes, using the mononitration of benzene as an example Understand that reactions of arenes, such as those in point 2 above, are used by industry during the synthesis of commercially important materials, for example explosives, pharmaceuticals and dyes (from nitration), and polymers such as polystyrene (from alkylation), Chapter 2 Objectives (continued) 5. 6. 7. 8. Explain the relative resistance to bromination of benzene, compared with cyclohexene, in terms of delocalisation of the benzene ring. Describe the reactions of phenol with bases and sodium to form salts and with bromine to form 2,4,6-tribromophenol Explain the relative ease of bromination of phenol, compared with benzene, in terms of activation of the benzene ring State the uses of phenols in antiseptics and disinfectants HC CH HC CH HC Consider only the carbon ring that lies in a plain. The structure of benzene can be represented in a variety of ways CH C C C C C C Add the bonds noticing they are adding perpendicular to the plain of the ring, then taking away the lower lobes for clarity If you imagine the lobes being large enough to overlap, the image changes to: Again for clarity, the electron clouds above and below the plain of the ring have been reduced in size , the green being above the plain of the ring and pink below. e- C e- C e- C C e- e- C C Because of the delocalisation, a benzene ring does not attract electrophiles with the same force as aliphatic double bond molecules. e•Three electrons are added to each cloud area. •Because of the overlapping orbitals, they are able to move over the entire perimeter of the ring. •This is known as delocalisation. Replay slides Electrophilic Substitution of Arenes • Example 1: A mixture of concentrated nitric acid (HNO3) and concentrated sulphuric acid (H2SO4) The sulphuric acid catalyst provides protons H2SO4 (l) H+ + HSO4- Nitric acid has this spatial arrangement. The proton from H2SO4 adds to HNO3 creating a Rearrangement new arrangement then occurs HNO3 This is the electrophile which will add molecules with NO2+ double bonds. Nitronium ion To replay the sequence, right click on the screen and select previous. Loss of H2O occurs Substitution on the benzene ring continues as illustrated benzene nitronium ion NO2+ A pair of electrons moves from the ring toward the nitronium ion… The nitronium ion moves toward the benzene ring giving the intermediate Loss of H+ occurs finally giving nitro benzene To replay the sequence, right click on the screen and select previous. Electrophilic Substitution of Arenes • Example 2: A halogen (X2) in the presence of a Iron (III) chloride arrives halogen carrier. (Br2 with FeCl3) as the catalyst to help the reaction. Benzene… Initially, bromine does not become polarized enough to react with benzene. In the presence of the catalyst, bromine’s polarity changes from… + Br Br Br Br2Br …is going to react with bromine _ FeCl Fe 3Cl Cl Cl Br Fe Cl Cl _ + Electon density shifts… Into… Bromine’s electron density is immagined as… Cl _ + Br+ _ It spatial arrangement creates the following dipoles… To create… Answer SAQ 2.1, 2.2 & 2.3 Br+ ion is a strong enough electrophile to add to the benzene ring The hydrogen atom is substituted by the bromine atom Br+ Br H + HBr Cl + Br FeCl Fe 3 Cl Cl The catalyst is regenerated To replay the sequence, click Phenols and their properties OH Phenol occurs widely in nature but the effects differ remarkably. OH Estradiol HO •An important female sex hormone •Maintains female sexual characteristics •Stimulates RNA synthesis and therefore promotes growth. Phenol OH O CH 3 •Found in seed pods of vanilla orchid •Used as flavouring additive in ice cream and chocolate C H H2 C H2C O Cholesterol Vanillin CH 2 H2C CH 3 CH CH 3 HO Phenol reacting with Alkali (NaOH) OH The ring draws electron density toward it This weakens the O-H bond allowing it dissociate more easily than other alcohols. It is therefore slightly acidic. pH ~ 6 + + - O Na+ + OHNa NaOH Sodium phenoxide An alkali will react with phenol in the expected way producing a salt and water. + H2O Phenol reacting with sodium metal (Na(s)) OH + 2Na 2 O Na Na(s) reacts similarly with all alcohols… …but phenol is somewhat more reactive. Because density is drawn into the ring… …the hydrogen comes off more readily (H+) Reduction: gain of electrons Sodium metal and hydrogen ions undergo oxidation and reduction. Na(s) + 2H+(aq) Oxidation: loss of electrons Na+(aq) + H2(g) + H2 (g) OH OH Br 1 Br HBr Br Br2 Br 1 H OH OH Br Br Br Br Br 2 HBr 2 H Br OH OH Br Br Br 3 Br HBr Br Br H Br Br 3 OH OH Br + phenol Br + 3Br2 bromine Br 2,4,6-tribromophenol Answer SAQs 2.4, 2.5 & 2.6 3HBr Hydrogen bromide CH3 CH 3 SAQ 2.1 A. Draw and name three isomers which might be produced following electrophilic substitution of NO2+ for one hydrogen atom in methylbenzene. NO 2 NO 2 2-nitro methyl benzene 3-nitro methyl benzene CH 3 NO 2 4-nitro methyl benzene B. TNT has the systematic name 1-methyl-2,4,6trinitrobenzene. Draw the structural formula of TNT CH 3 O2N NO 2 NO 2 2,4,6-trinitro methyl benzene SAQ 2.3 A. Suggest a suitable halogen carrier to use in the reaction of benzene with chloromethane. B. Suggest suitable reactants which might lead to the formation of the following compound in the presence of a halogen carrier. Anhydrous FeCl3 or AlCl3 Benzene + 2-chloro-2-methyl propane CH 3 + H3C C CH 3 Cl B. Write a balanced equation using your suggested reactants CH 3 + Cl C CH 3 CH 3 CH 3 CH 3 CH 3 SAQ 2.6 Br A. How does bromine in aqueous solution become sufficiently polar to achieve electrophilic substitution _ Fe FeCl3 Br + Br Br Br Bromine has a symmetrical electron density arrangement _ _ In the presence of FeBr3, the electron density shape changes