in the room until the thermal equilibrium is established. The... 7-101
advertisement
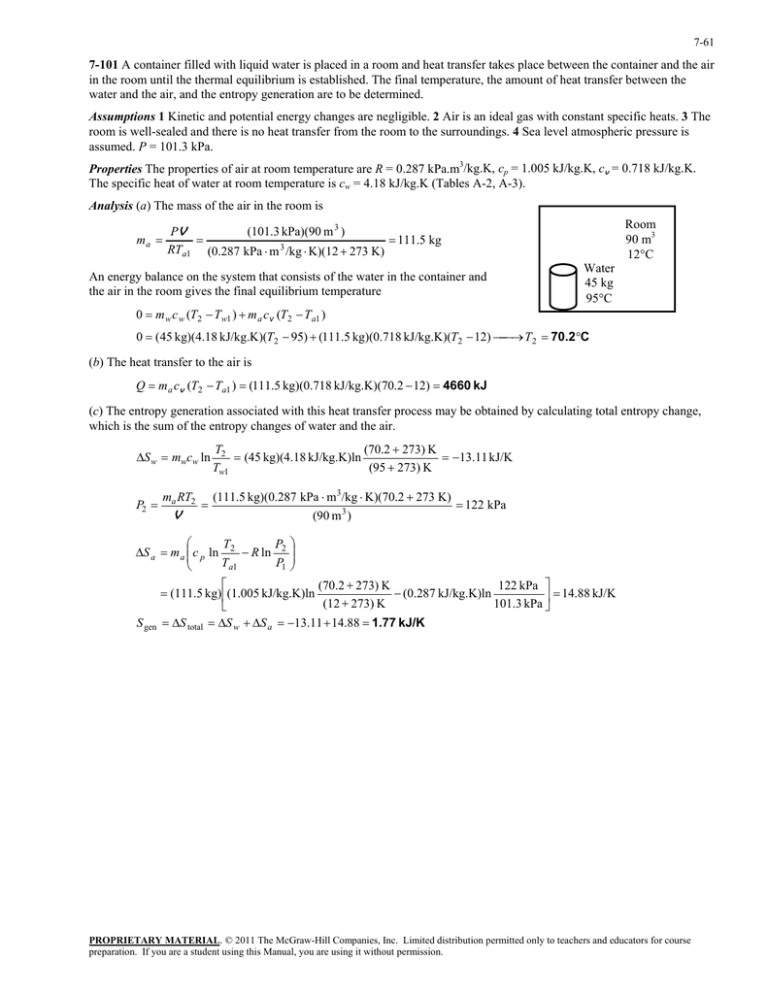
7-61 7-101 A container filled with liquid water is placed in a room and heat transfer takes place between the container and the air in the room until the thermal equilibrium is established. The final temperature, the amount of heat transfer between the water and the air, and the entropy generation are to be determined. Assumptions 1 Kinetic and potential energy changes are negligible. 2 Air is an ideal gas with constant specific heats. 3 The room is well-sealed and there is no heat transfer from the room to the surroundings. 4 Sea level atmospheric pressure is assumed. P = 101.3 kPa. Properties The properties of air at room temperature are R = 0.287 kPa.m3/kg.K, cp = 1.005 kJ/kg.K, cv = 0.718 kJ/kg.K. The specific heat of water at room temperature is cw = 4.18 kJ/kg.K (Tables A-2, A-3). Analysis (a) The mass of the air in the room is ma Room 90 m3 12C (101.3 kPa)(90 m 3 ) PV 111.5 kg RTa1 (0.287 kPa m 3 /kg K)(12 273 K) An energy balance on the system that consists of the water in the container and the air in the room gives the final equilibrium temperature Water 45 kg 95C 0 m w c w (T2 Tw1 ) m a cv (T2 Ta1 ) 0 (45 kg)(4.18 kJ/kg.K)(T2 95) (111.5 kg)(0.718 kJ/kg.K)(T2 12) T2 70.2C (b) The heat transfer to the air is Q m a cv (T2 Ta1 ) (111.5 kg)(0.718 kJ/kg.K)(70.2 12) 4660 kJ (c) The entropy generation associated with this heat transfer process may be obtained by calculating total entropy change, which is the sum of the entropy changes of water and the air. S w mwcw ln P2 ma RT2 V (70.2 273) K T2 (45 kg)(4.18 kJ/kg.K)ln 13.11 kJ/K (95 273) K Tw1 (111.5 kg)(0.287 kPa m3/kg K)(70.2 273 K) (90 m3 ) 122 kPa T P S a m a c p ln 2 R ln 2 T P1 a1 S gen (70.2 273) K 122 kPa (0.287 kJ/kg.K)ln (111.5 kg) (1.005 kJ/kg.K)ln 14.88 kJ/K (12 273) K 101.3 kPa S total S w S a 13.11 14.88 1.77 kJ/K PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.








