Understanding Risk and Resilience Factors for Suicide
advertisement

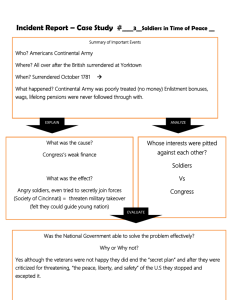
ARMY STARRS/STARRS LS Understanding Risk and Resilience Factors for Suicide Robert J. Ursano, M.D. Prof/Chair Dept of Psychiatry Uniformed Services University Director Center for the Study of Traumatic Stress Disclosures • I have no relevant financial relationship with the manufacturers of any commercial products and/or providers of commercial services discussed in this CME activity. • Neither I nor any member of my immediate family has a financial relationship or interest with any proprietary entity producing health care goods or services related to the content of this CME activity. • My content will not include reference to commercial products. • I do not intend to discuss any unapproved or investigative use of commercial products or devices. Trauma and Disasters Human Made Natural Industrial Accident Hurricane War Terrorism Epidemic Suicide State of Knowledge and Need “Suicide is among the leading causes of death and disease burden around the world. Although there have been significant advances in suicide research as well as increases in the treatment of suicidal people, the rate of suicidal behaviors has not changed as a result” Nock M, et al WHO PLoS 2009 Count and Crude Suicide Rates Among Active Duty and Reserve Service Members Mortality Surveillance Div, AFMES, AFIP 2010; DoD Suicide Prev Task Force Psychiatric Responses to Trauma Distress Responses • Anxiety • PTSD • Depression • Resilience Mental Health/ Illness • Change in Sleep • Decrease in Feeling Safe • Isolation (staying at home) Health Risk Behaviors (changed behavior) • Smoking • Alcohol • Over dedication •Change in travel •Separation anxiety The Past… • “One type of symptomatic behavior associated with depressions, either neurotic or psychotic in type, is suicide. Between July, 1940, and June 1946, there were 2,214 suicides in the Army, 300 of which occurred among officers.1 ….these figures represent a sharp drop during the war period from the peacetime suicide rate in the Army. 2 There was also a sharp drop in the number of suicides in the Army in World War “ Menninger, K. Psychiatry in a Troubled World. Pp. 166-167, 1948 Suicidal Thoughts and Behavior in the Past Year among Adults Aged 18 or Older: 2008 0.9 Million Made Plans and Attempted Suicide 2.3 Million Made Suicide Plans . . . 8.3 Million Adults Had Serious Thoughts of Committing Suicide 1.1 Million Attempted Suicide 0.2 Million Made No Plans and Attempted Suicide (SAMHSA, 2008) Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) Co-Principal Investigators: Robert J. Ursano MD (USUHS) Murray B. Stein MD, MPH (UCSD) on behalf of the Army STARRS Research Team May 4, 2015 Slide 9 Challenges • • • • • • • • • Across agency MOUs (Army, NIMH) Civilian and DoD Scientists Collaboration (HJF) Confidentiality (Certificate/Letter from Sec of Army) Disclosure Multi Site “In Theater” Quarterly reports to senior leadership GWAS Complex internal organization Slide 10 Suicide Trends for Active Duty Army & Matched Civilians * Crude suicide rates, using data from Army STARRS (2004-2009) and Army Active Duty Strength reports (2010-2013) ** Civilian data from Centers for Disease Control, adjusted by Army STARRS to 2004 Army distribution of age, sex and race/ethnicity Slide 11 Active Duty Army Suicide Rates Source: Army STARRS calculations Slide 12 Administration & Funding • • • • Army STARRS is being conducted by a consortium of investigators from the Uniformed Services University of the Health Sciences (USUHS), the University of California-San Diego (UCSD), Harvard Medical School (HMS) & the University of Michigan (UM) in collaboration with the NIMH. Co-PIs: R. Ursano (USUHS) & M. Stein (UCSD) Site-PIs: R. Kessler (HMS) & S. Heeringa (UM) Collaborating NIMH Scientists: L. Colpe & M. Schoenbaum Consulting Army Scientists: K. Cox & S. Cersovsky Supported by NIH Cooperative Agreement U01MH87981. Funding provided by the Department of the Army ($50M) with supplemental funds ($15M) from the National Institute of Mental Health (NIMH). Army STARRS in-theater research was conducted under a protocol reviewed and approved by the U.S. Army Medical Research and Materiel Command (MRMC) Institutional Review Board, and in accordance with the approved protocol. Slide 13 Background and Goals Background: The persistent rise in Army suicide rate led the Army to ask NIMH to find academic scientists with the expertise to design & conduct an independent research program that was large, creative, and comprehensive enough to address this complicated problem. Goals: To identify risk and protective factors that impact Soldiers’ well-being so the Army can use them in risk reduction efforts. • Identify salient risk and protective factors in Army Soldiers • Inform development & testing of empirically-derived interventions for Army Soldiers • Deliver “actionable” findings to the Army rapidly • Establish Army cohorts for future follow-up studies & continued benefit to the Army Slide 14 Study Design • Army STARRS is not a single study • Integrated multi-study design • Involves seven epidemiologic & neurobiologic studies to comprehensively investigate risk factors & protective factors for: – Suicide – Suicide-related behavior – Related mental and behavioral health Slide 15 Overview of Studies Study Overview and Status Historical Administrative Data Study (HADS) • • • • To compile, organize & analyze existing administrative Army and DoD data. Involves huge volume of data, enormous effort, allows analyses never before possible. Includes more than 1.6 million active duty Soldiers from 2004 to 2009. Includes more than 1.1 billion de-identified Army/DoD records from ~40 sources. New Soldier Study (NSS) • • • • • • To assess health, personal characteristics, and prior experiences of new Soldiers. Data collected at 3 sites: Ft. Jackson, Ft. Benning & Ft. Leonard Wood. Data collection began Feb 2011 and ended Nov 2012. Blood collection began Sep 2011 and ended Nov 2012. ~57,000 Soldiers attended survey sessions. ~35,000 gave blood (80% of those asked). All Army Study (AAS) In-Theater AAS (Kuwait) • To assess Soldiers across all phases of Army service. • Data collection began Jan 2011 and ended Apr 2013. • ~35,000 Soldiers attended survey sessions at >50 CONUS and OCONUS sites. • • • • To include in-theater Soldiers in the AAS. Data collected from both “outbound” and “inbound” Soldiers during R&R processing. Data collection began Mar 2012 and ended Sep 2012. ~10,000 Soldiers attended survey sessions. Slide 16 Overview of Studies (Continued) Study Overview and Status Soldier Health Outcomes Study A (SHOS-A) • To assess Soldiers who were hospitalized for a suicide attempt (cases) and compare to control Soldiers from the AAS. • 5 Military Treatment Facilities: JBLM, Walter Reed, Ft. Hood, Ft. Bragg & Ft. Stewart. • Data collection began Nov 2011 and ended Dec 2013. • Target enrollment: 450 Soldiers (~150 cases and ~300 controls). • Actual enrollment: 561 Soldiers (186 cases and 375 controls). • 296 Soldiers provided blood samples. Soldier Health Outcomes Study B (SHOS-B) • To assess Soldiers who committed suicide (cases) and compare to control Soldiers from the AAS. • Interviewing Army supervisors and next-of-kin of cases and controls. • Data collection began Mar 2012 and ended Jan 2014. • Target enrollment: 560 total interviews for ~135 cases and ~270 controls. • Actual enrollment: 603 interviews completed for 150 cases and 276 controls. • By far the largest psychological autopsy study of a military population, and one of the largest suicide psychological autopsy studies ever done. Slide 17 Overview of Studies (Continued) Study Overview and Status Pre/Post Deployment Study (PPDS) • To follow deploying Soldiers over time to assess the effects of deployment. • Longitudinal study of 3 BCTs: JBLM, Ft. Bragg & Ft. Carson. • Four waves (time-points) of data collection: 1 pre-deployment & 3 post-deployment 1. Pre Time 0 (T0) at ~2-3 weeks before deployment (survey and blood). 2. Post Time 1 (T1) at ~2-3 weeks following redeployment (survey and blood). 3. Post Time 2 (T2) at ~2-3 months following redeployment (survey). 4. Post Time 3 (T3) at ~8-9 months following redeployment (survey). • Data collection began Jan 2012 and ended Apr 2014. • T0 data collection : ~9,500 Soldiers (~8,000 Soldiers provided blood samples). • T1 data collection: ~10,000 Soldiers (~8,800 Soldiers provided blood samples). • T2 data collection: ~9,200 Soldiers. • T3 data collection: ~7,000 Soldiers. Clinical Reappraisal Study (CRS) • To calibrate clinical survey measures used in AAS and NSS. • Calibration interviews began Mar 2012 and ended Nov 2012. • 460 Soldiers participated. Slide 18 Data Collection Timeline July 2009 to June 2014 Year 1 Year 2 Year 3 Year 4 Year 5 July 2009-June 2010 July 2010-June 2011 July 2011-June 2012 July 2012-June 2013 July 2013-June 2014 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Design, Develop, Pre-Test Research Data Enclave and Historical Administrative Data Study (HADS) New Soldier Study (NSS) All Army Study (AAS) CRS Soldier Health Outcomes Study A (SHOS-A) Soldier Health Outcomes Study B (SHOS-B) PPDS T0 PPDS T1 PPDS T2 PPDS T3 Slide 19 AAS Data Collection Locations Slide 20 Locations of All Collaborators Principal Investigators, Other Investigators, Scientific Advisory Board Members, Labs & Vendors Slide 21 Data Collection Summary: Soldiers, Surveys, Blood Samples For Studies with Data Collection from Soldiers (HADS not included) Approx. Number of Blood Samples Approx. Number Approx. Number Study of Soldiers Who of Surveys Soldiers Who Blood Tubes Blood Vials in Participated Collected Provided Blood Collected Frozen Storage NSS (2 sessions/Soldier) 57,000 114,000 35,000* 35,000 35,000 AAS (incl. Guard & Reserve) 35,000 35,000 AAS Kuwait (in-theater) 10,000 10,000 PPDS pre Time 0 9,500 8,000 24,000 55,000 PPDS post Time 1 10,000 8,800 17,500 57,000 10,000 PPDS post Time 2 9,200 PPDS post Time 3 7,000 SHOS-A 186** 1,200 300 600 600 SHOS-B 150** 600 APPROXIMATE TOTAL 112,000 196,000 52,000 77,000 148,000 * NSS blood collection was added 6 months after study began. Approx. 80% of Soldiers who were asked gave blood. ** Cases Only -- Controls are already counted in AAS Slide 22 Biomarkers Summary Analysis Types and Sample Sizes 1. GWAS: ~16,000 Soldiers • ~8,000 NSS Soldiers • ~8,000 PPDS Soldiers (pre-deployment) • Custom chip (whole exome arrays plus ~6K custom features) 2. DNA Methylation: ~400 PPDS Soldiers (pre & post deployment) 3. DNA Telomeres: ~400 PPDS Soldiers (pre & post deployment) 4. RNA Expression: >200 PPDS Soldiers (pre & post deployment) 5. BDNF/Cytokines: ~400 PPDS Soldiers (pre & post deployment) 6. Metabolites: ~250 PPDS Soldiers (pre & post deployment) 7. Genotyping: ~2,800 NSS Soldiers (PsychChip) 8. Genotyping: ~300 SHOS-A Soldiers (PsychChip) Slide 23 Approach to Producing Actionable Findings Concentration of Risk • Who (e.g., MOS, rank, demographics, mental disorders) • When (e.g., time in service, deployment status, time pre/post deployment) • Where (e.g., installations, training, combat zones, transitioning) Risk variables • Identify risk sub-groups (who, when, where) so Army can consider programs to target for intervention Neurocognitive • Use neurocognitive tests to identify those at risk and possible neurocognitive functioning associations with suicidal behavior Biomarkers • Identify biomarkers for those at risk and determine possible neurobiologic mechanisms Slide 24 SELECTED PUBLISHED/PRESENTED FINDINGS Slide 25 Suicidal Thoughts/Behaviors among New Soldiers (NSS) Suicide Ideation Suicide Plan Suicide Attempt Lifetime Past 30 Days 13.5% 1.1% (n=6,192) (n=473) 2.7% 0.1% (n=1,234) (n=42) 1.9% 0.1% (n=878) (n=58) Slide 26 Risk of Suicide Attempt by Month Since Entering Army, Enlisted Soldiers & Officers (HADS, 2004-2009, n=193,617) The sample of 193,617 person-months includes all Regular Army soldiers (i.e., excluding those in the U.S. Army National Guard and Army Reserve) with a suicide attempt in the administrative records during the years 2004-2009, plus a 1:200 stratified probability sample of all other active duty Regular Army person-months in the population exclusive of soldiers with a suicide attempt or other nonfatal suicidal event (e.g., suicidal ideation) and person- months associated with death (i.e., suicides, combat deaths, homicides, and deaths due to other injuries or illnesses). All records in the 1:200 sample were assigned a weight of 200 to adjust for the undersampling of months not associated with suicide attempt. Slide 27 Examples of Recent Findings from Historical Administrative Data Study 2004-2009 Historical Administrative Data Study (>975K regular Army Soldiers in 2004-2009) • Suicide risk increased for those deployed, never deployed, and previously deployed; deployed & previously deployed at greater risk. • Suicide risk lower for females than males (as with civilians), but difference narrowed substantially during deployment. • Suicide risk was increased for those demoted in past 2 years. • Currently & previously deployed in first 4 years of service had greater risk than never deployed. • Not associated with increased risk of suicide: o o o o Waivers Length of time since return from most recent deployment Total number of deployments Interval between 2 most recent deployments (dwell time) Schoenbaum, et al. JAMA Psychiatry, 2014 Slide 28 TBI AND SUICIDE RISK Slide 29 Age at First TBI (in AAS Q2-Q4) (M. Stein, et al) Slide 30 Multivariate model predicting suicidality1 (M. Stein, et al) Lifetime Suicide Ideation Lifetime Suicide Plan Lifetime Suicide Attempt OR [95% CI] OR [95% CI] OR [95% CI] Antecedent TBI1 1.7 [1.4-2.0] 1.9 [1.5-2.5] 1.6 [1.2-2.2] Antecedent TBI2 (full model) 1.4 [1.2-1.6] 1.6 [1.1-2.1] 1.3 [0.9-1.8] 1 Multivariate model predicting suicidality outcomes with TBI (0,1,2) controlling for all demographics and interaction between "not entered Army yet" and "birth place"; controlling for years since ideation for outcomes among ideators. 2 As above and controlling for mental disorders. Note PARP for TBI 20-30% Slide 31 Mental Health and Suicide Slide 32 AAS:Thirty-Day Prevalence of DSM-IV Mental Disorders among Nondeployed Soldiers in the U.S. Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) • • • • • • • A total of 25.1% of respondents met criteria for any 30-day disorder (15.0% internalizing; 18.4% externalizing) and 11.1% for multiple disorders. A total of 76.6% of cases reported pre-enlistment age at onset of at least one 30-day disorder (49.6% internalizing; 81.7% externalizing). 12.8% of respondents reported severe role impairment. Controlling for sociodemographic and Army career correlates (which were broadly consistent with other studies) 30-day disorders with pre-enlistment and postenlistment ages at onset both significantly predicted severe role impairment Pre-enlistment disorders were more consistent powerful predictors than postenlistment disorders. Population-attributable risk proportions of severe role impairment were 21.7% for preenlistment disorders, 24.3% for post-enlistment disorders, and 43.4% for all disorders. Interventions to limit accession or increase resilience of new soldiers with preenlistment mental disorders might reduce prevalence and impairments of mental disorders in the U.S. Army. Kessler, et al. JAMA Psychiatry, 2014 Slide 33 Concentration of Risk Inpatient: 28 to 263/100,000 to predictor 4/100 vs statins 7.5 ascvd/100 per 10 years Slide 34 Challenges • • • • • • • • • Across agency MOUs (Army, NIMH) Civilian and DoD Scientists Collaboration (HJF) Confidentiality (Certificate/Letter from Sec of Army) Disclosure Multi Site “In Theater” Quarterly reports to senior leadership GWAS Complex internal organization Slide 35