Unit-5-Lipids
advertisement

Chem 150
Unit 5 - Biological Molecules I
Lipids
Like organic molecules, biological molecules are grouped
into families. There are four major families of biological
molecules, including proteins, nucleic acids,
carbohydrates, and lipids. The lipids are the subject of this
unit. Of these four families, the lipids are the structurally the
most diverse. This is because unlike members of the other
three families, members of this families do no share a
common structural feature, but rather share a common
physical property; the are hydrophobic.
Introduction
Lipids are hydrophobic, nonpolar molelcules.
•
They are soluble in nonpolar solvent.
•
They are insoluble in polar solvents, such as water
They are isolated from the other biological molecules by
extracting them with nonpolar solvents.
2
Introduction
The types of lipids that we will look at include.
• Fatty Acids
•
•
•
•
•
•
3
•
In the carboxylic acid family
Waxes
•
Fatty Acids + Alcohols
Triglycerides
•
3 Fatty acids + glycerol
Phospholipids and glycolipids
•
2 fatty acids + glycerol + phosphate + X
Steroids
•
Derivatives of cholesterol
Eicosanoids
•
Derivatives of the Fatty acid arachidonic acid
Membranes
• Formed from phospholipids and glycolipids
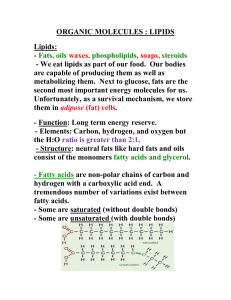
Fatty Acids
Fatty acids contain a carboxylic acid group
•
This should make them quite polar
However, they also contain a long hydrocarbon tail
•
Which overall, makes them nonpolar.
nonpolar
4
polar
Fatty Acids
Fatty acids typically contain between 12 and 20 carbons
•
•
The number is usually always even.
The nonpolar tails interact with London forces.
nonpolar
5
polar
Fatty Acids
Melting Temperature {°C}
Melting points for saturated fatty acids:
No. of Carbons
6
Fatty Acids
Some fatty acids contain double bonds
7
•
unsaturated
•
monounsaturated
•
polyunsaturated
•
polyunsaturated
Fatty Acids
The common fatty acids found in biological systems are
shown in Table 8.1 of Raymond.
Text
Linolenic acid is one of the omega-3 fatty acids.
8
Fatty Acids
Melting Temperature {°C}
Normally the double bonds are cis
• This lowers the melting points for fatty acids containing
double bonds.
No. of Double Bonds
9
Fatty Acids
The cis double bonds produce kinks, which disrupt the
London forces by preventing the tails from packing close to
one another.
10
Fatty Acids
As acids, the carboxylic acid group in fatty acids can react
with a base to produce a carboxylate ion
• By donating its proton (H+) to the base the fatty acid
becomes negatively charged.
• We will talk more about acids and bases in Unit 6
11
Fatty Acids
The negative charge makes the polar head portion of the the
fatty acid even more more polar and hydrophilic.
12
Fatty Acids
The salts of fatty acids are also called soaps, and are
considered amphipathic, meaning they have a part that is
very hydrophobic along with a part that is very hydrophilic.
•
13
In Unit 3 we discussed how amphipathic molecules form
interesting structures when exposed to water.
Biochemical Compounds &
Their Interactions with Water (Unit 3)
When placed in water,
amphipathic molecules,
form structures, such as
micelles, which attempt to
address the conflict.
14
Fatty Acids
The salts of fatty acids are also called soaps, and are
considered amphipathic, meaning they have a part that is
very hydrophobic along with a part that is very hydrophilic.
•
15
In Unit 3 we discussed how amphipathic molecules form
interesting structures when exposed to water.
Waxes
Waxes are made by combining fatty acids with long chain
alcohols.
• In Unit 2 we discussed how carboxylic acids react with
alcohols to from esters.
16
Alcohols, Carboxylic Acids & Esters (Unit 2)
We look now at three families that are distinguished by a
functional group that contains the element oxygen.
Esters
• Chemically, esters can be synthesize by reacting a
carboxylic acid with and alcohol:
O
CH3
CH2
Carboxylic
acid part
C
O
CH2 CH3
Alcohol
part
Ethyl propanoate
17
Waxes
Waxes are esters.
14-36 carbons
18
16-30 carbons
Waxes
When two more molecules combine to form a larger
molecule, the word residue is used to indicate which
molecule that part of the the larger molecule came from.
came from the fatty acid
19
came from the alcohol
Waxes
Waxes are very hydrophobic and are used by plants and
animals for protective, water-proof coatings
20
Questions
Draw the skeletal structures for the products formed when
beeswax undergoes base-catalzyed hydrolysis
(saponification).
21
Reactions Involving Water (Unit 4)
Hydrolysis
• Hydrolysis can also be catalyzed using a base (OH-):.
O
CH3
CH2 CH2 C
O
O
CH2 CH3
ethylbutanoate
(an ester)
22
H2O
OH-
CH3
CH2
CH2
C
O
butanoate ion
(a carobxylate ion)
+
HO
CH2 CH3
ethanol
(an alcohol)
•
Because one of the products of the hydrolysis is a carboxylic acid, in base
catalyzed hydrolysis the base undergoes a second acid/base reaction with the
carboxylic acid to produce a carboxylate ion.
•
The base catalyzed hydrolysis of esters is also called saponification
•
We will be discussing acids and bases in Unit 6
Triglycerides
Triglycerides are a storage form of fatty acids in mammals.
•
Often when blood tests are done, they measure your
triglycyeride levels.
•
High triglyceride levels in the blood are a risk indicator for
artherosclerosis.
*American Heart Association
23
Triglycerides
Triglycerides are a combination of three 3 fatty acid
molecules with a glycerol molecule.
24
Triglycerides
Glycerol, which is also called glycerin,
is an alcohol with three hydroxyl
groups.
•
•
As with the waxes, the fatty acids
can react with the hydroxyl groups to
form esters.
Since there are three hydroxyl
groups, three fatty acids can react to
form three esters.
HO
CH2
HO
CH
HO
CH2
glycerol
25
Triglycerides
For triglycerides, all three hydroxyls of the glycerol have a
fatty acid residue attached to it.
O
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2C
O
CH2
O
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2C O
CH
O
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2C O
fatty acid
residues
26
CH2
glycerol
residue
Figure 8.6
from
Raymond
27
Triglycerides
Just as with fatty acids, where the presence of cis double
bonds lower the melting points, triglycerides made from
unsaturated fatty acids have lower melting points than those
made from saturated fatty acids.
28
•
Triglycerides from animals tend to have a higher proportion
of saturated fatty acids.
• Most are solids at room temperature and are called fats.
• Examples include: butter, lard and bacon grease
•
Triglycerides from plants tend to have a higher proportion
of unsaturated fatty acids.
• Most are liquids at room temperature and are called oils.
• Examples include: corn oil, canola oil, peanut oil and olive oil.
Triglycerides
Triglycerides as primarily used as a form of stored energy.
•
This is why when you eat more than you need to meet your
energy requirements, the excess energy is stored in the
form of fat.
•
29
Fat can store almost twice as much energy per gram as
carbohydrates and proteins
•
In mammals the fats are stored in the adipose tissue.
•
Adipose tissue also functions to protect organs from shock
and cold.
Triglycerides
Reactions that involve triglycerides include:
•
•
•
30
Hydrogenation
Oxidation
Base-catalyzed hydrolysis (saponification)
Transport of fats:
LDL transfers
cholesterol to
tissues from liver.
Dietary fat
31
VLDL moves triglycerides
from liver to tissues.
HDL carries
cholesterol from
tissues to liver.
Monoglycerides and fatty
acids are absorbed by
intestines - transported
as chylomicrons in lymph
system to blood.-Fat
Blocker-Xenical (Alli)
Transport of fats:
Fat Blocker-Xenical (Alli)
Typical diacylglycerol
O
O
OH
O
O
32
Fun Topic !: Fake Fats
Side effects?
33
Olestra: sucrose
fatty acid ester(s)
Fat : glycerol fatty
acid ester(s)
Triglycerides
Hydrogenation of triglycerides
•
This is the same reaction that we saw in Unit 4 with the
hydrogenation of alkenes.
• Unsaturated fats and oils contain alkenes and can be hydrogenated
to produce saturated fats.
•
Commercially, vegetable oils are often hydrogenated to produce a
solid product that has better qualities for making baked goods.
‣ Animal fats, such as butter and lard, which are naturally saturated, can
also be used, but unlike the vegetable oils, they come with cholesterol,
which is undesirable for health reasons.
34
Oxidation and Reduction (Unit 4)
Hydrogenation
• Another type of oxidation/reduction reaction is the
hydrogenation reaction:
•
In this example, an alkene is reduced to an alkane.
‣
•
35
H
H
H
H
This isCconsidered
reduction,
because the hydrogenHis bringing
in
+
H
C
C
C additional
H
2
platinum
electrons to the molecule.
H
H
catalyst
H
H
The alkane that is produced in this reaction is considered “saturated” because it
can no
longer absorb any more hydrogen atoms.
unsaturated
saturated
Triglycerides
Hydrogenation of triglycerides
•
This is the same reaction that we saw in Unit 4 with the
hydrogenation of alkenes.
•
Unsaturated fats and oils contain alkenes and can be
hydrogenated to produce saturated fats.
•
Commercially, vegetable oils are often hydrogenated to
produce a solid product that has better qualities for making
baked goods.
• Animal fats, such as butter and lard, which are naturally saturated,
can also be used in baking, but unlike the vegetable oils, they come
with cholesterol, which is undesirable for health reasons.
36
Triglycerides
Hydrogenation of triglycerides
•
Total hydrogenation
liquid
37
solid
Triglycerides
Hydrogenation of triglycerides
•
Partial hydrogenation
liquid
38
solid
Triglycerides
Hydrogenation of
triglycerides
•
Partial
hydrogenation
cab produce
trans fats.
• Trans fats have
been found to
lower your HDL
(“Good
cholesterol”)
levels.
39
Triglycerides
Saturated vs Unsaturated Fats
40
Triglycerides
Saturated vs Unsaturated Fats
41
Triglycerides
Saturated vs Unsaturated Fats
42
Triglycerides
Saturated vs Unsaturated Fats
43
Triglycerides
Saturated vs Unsaturated Fats
saturated fat
O
C
trans unsaturated fat
H
O
CH2
O
C
O CH
H
cis unsaturated fat
H
H
44
O
C
O CH2
Fat (Triacylglyceride)
Triglycerides
Oxidation of triglycerides
•
•
45
Unsaturated triglycerides can react with oxygen to produce
small change fatty acids another small molecules.
•
These often do not smell very good
•
This is what happens when butter goes rancid.
This makes solid fats and oils more stable than liquid oils
and is why the solid fats are preferred for deep frying.
Triglycerides
Oxidation of triglycerides
These stink !
46
Triglycerides
Saponification of triglycerides
•
Saponification is the base-catalyzed hydrolysis of the ester
bonds in a triglyceride.
•
We also discussed this reaction in Unit 4
•
This cleaves the esters back into carboxylic acids (fatty
acids) and an alcohol (glycerol).
•
Because the reaction is base-catalyzed, the base also
reacts with the carboxylic acids to from carboxylate ions
•
47
We saw this on an earlier slide
Reactions With Water (Unit 4)
Hydrolysis example:
• The base catalyzed hydrolysis of fats produces soap and
glycerol
O
C
H
O
CH2
O
C
3 H2O
O CH
H
H
H
O
C
Fat
48
O CH2
OH-
Reactions With Water (Unit 4)
Hydrolysis example:
• The base catalyzed hydrolysis of fats produces soap and
glycerol
O
C
O
HO
CH2
HO
CH
HO
CH2
O
H
3 H2O
C
O
OH-
+
H
H
O
H
C
O
Soap
49
Glycerol
Phospholipids and Glycolipids
Phospholipids and Glycolipids are the stuff that biological
membranes are made of.
•
50
Like the soaps, these molecules are highly aphipathic, and
when mixed with water spontaneously form membranes
that are described as lipid bilayers.
Phospholipids and Glycolipids
Soaps form
Micelles
51
Phospholipids form
Lipid Bilayers
Phospholipids and Glycolipids
Phospholipids and Glycolipids are the stuff that biological
membranes are made of.
•
52
Like the soaps, these molecules are highly aphipathic, and
when mixed with water spontaneously form membranes
that are described as lipid bilayers.
Phospholipids and Glycolipids
Phosphospholipids
•
53
There a are two types of phospholipids
• Glycerophospholipids
Phospholipids and Glycolipids
Phosphospholipids
•
54
There a are two types of phospholipids
• Sphingolipids
Phospholipids and Glycolipids
Phosphospholipids
•
The Glycerophospholipids have a structure similar to
triglycerides, with one of the fatty acids replaced with a
phosphate.
There is usually
an additional
alcohol attached
to the other side
of the phosphate
55
Phospholipids and Glycolipids
Phosphospholipids
•
The Glycerophospholipids have a structure similar to
triglycerides, with one of the fatty acids replaced with a
phosphate.
phosphoester
56
bonds
Phospholipids and Glycolipids
Phosphospholipids
•
The Glycerophospholipids have a structure similar to
triglycerides, with one of the fatty acids replaced with a
phosphate.
“Phosphotidyl-”
refers to everything
but the X
57
Phospholipids and Glycolipids
Phosphospholipids
•
Phospholipids are used commercially as emulsifying
agents.
• An emulsifying agent stabilizes an emulsion.
• An emulsion is a colloidal suspension of one liquid in another.
‣ An example is mayonnaise, which is a colloidal suspension of oil and
water.
•
58
Lecithin, which is another name for the phospholipid
phosphotidylcholine, is used as an emulsifying agent in
mayonnaise and other prepared foods.
Phospholipids and Glycolipids
Phosphospholipids
•
The sphingolipids function similarly to the
glycerophospholipids, but structurally they are different.
• There is not glycerol core
• The glycerol and one of the fatty acids found in glycerophospholipids
is replaced with a molecule called sphingosine.
59
Phospholipids and Glycolipids
Phosphospholipids
60
•
The sphingolipids are found in the myelin membranes that
insulate the nerve cells.
•
Some sphingolipids use sugars for the alcohol portion of
the molecule
• These are called glycolipids.
Steroids
Steroids are a type of lipid that is not derived form a fatty
acid.
• They are based instead on a system of five cycloalkane
rings that are fused together.
61
Steroids
Steroids are a type of lipid that is not derived form a fatty
acid.
• They are based instead on a system of five cycloalkane
rings that are fused together.
62
Steroids
Cholesterol is the steroid that used as the starting point for
the synthesis of other steroids.
Note the fused ring
system
63
Steroids
•
•
Cholesterol is only found in animals
Besides being used to synthesize the other steroids,
cholesterol is dissolved in membranes to keep them fluid.
• Plants use the alternative strategy of using polyunsaturated fatty
acids to make their phospholipids.
64
Lipoproteins
Lipoproteins are used to transport
the water insoluble lipids such as
triglycerides, phospholipids and
cholesterol, in the blood.
• Lipoproteins contain lipids and
proteins.
• They include:
• Chylomicrons transport primarily
•
•
65
triglycerides from the digestive track.
LDLs (low density lipoproteins)
transport cholesterol, triglycerides and
phospholipids from the liver to other
tissues.
HDLs (high density lipoproteins)
transport cholesterol and
phospholipids back to the liver.
Lipoproteins
The HDL and LDL levels in the blood can be used to assess
ones risk for atherosclerosis.
•
•
66
High levels of HDL is considered good
•
This is why HDL is sometimes referred to as “good cholesterol”
•
> 40 mg/dL is good.
High levels of LDL is considered bad
•
This is why LDL is sometimes referred to as “bad cholesterol”
•
> 100 mg/dL is bad.
67
Eicosanoids
Eicosanoids are derived from arachidonic acid:
68
Membranes
Fluid mosaic model
69
Membranes
Transport across membranes
70
The End