Molecular Shape
advertisement
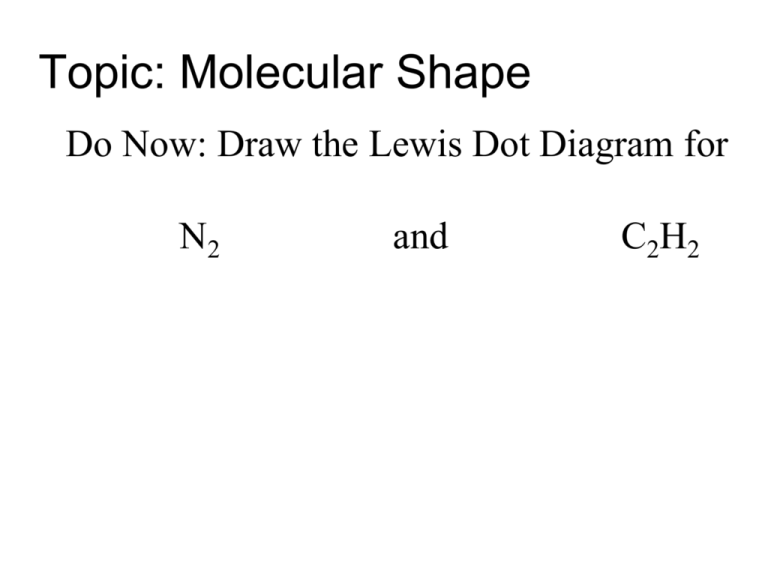
Topic: Molecular Shape Do Now: Draw the Lewis Dot Diagram for N2 and C2H2 BONDING Capacity ELEMENT # of bonds it likes to make Halogen,F, Cl, Br, I 1 Hydrogen 1 O and S 2 N and P 3 C 4 Molecular Shape H O H Use the Lewis Structure • Lewis structure is 2-D, but can help figure out 3-D shape Molecular Shape • Shape determined by two factors: 1. # of unpaired e- around the central atom 2. total # atoms bonded to central atom It’s important to classify electron pairs as bonding or nonbonding • Electron pairs repel each other: • want to be as far apart from each other as can be • Nonbonding pairs take up a little more room than bonding pairs 2-Atom Molecules • Atoms located right next to each other • linear molecules! 3-Atom Molecules • linear or bent Bent Linear H2 O .. Lewis Structure of H2O = H:O:H .. 3 total atoms, free unpaired electrons = BENT CO2 .. .. Lewis structure: O :: C :: O .. .. 3 total atoms, no free unpaired electrons = Linear 4 atom molecules They make triangles… …so we call them trigonal Planar or pyramidal Trigonal Pyramidal = Free electrons around the central atom 4-Atom Molecules: Trigonal Planar = no free electrons around the central atom 4-Atom Molecules: Planar 5-Atom Molecules: H .. H:C : H .. H Tetrahedral Molecular Polarity Look at structure and shape of molecule - all bent molecules are polar Draw a • If top/bottom and left/right are same then NONPOLAR • If either of the two are different then POLAR Symmetrical Molecules Nonpolar – they don’t have poles Asymmetrical Molecules Polar – they have poles (different ends) Let’s try a few… Name the SHAPE and POLARITY BENT POLAR Trigonal Planar POLAR Tetrahedral POLAR Trigonal Pyramidal POLAR linear POLAR linear POLAR bent POLAR tetrahedral NONPOLAR







