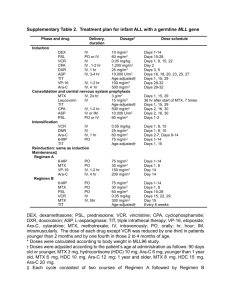
Guidelines in the Management of RA
advertisement

Guidelines in the Management of RA Haya M. Al-Malaq Lecturer, Clinical Pharmacy Dept. Clinical Pharmacist Rheumatology Haya_malak@yahoo.com Introduction • Rheumatoid arthritis (RA) is an autoimmunedisorder of unknown etiology characterized by symmetric, erosive synovitis and, in some cases, extraarticular involvement. Most patients experience a chronic fluctuating course of disease that, despite therapy, may result in progressive joint destruction, deformity, disability, & even premature death. Epidemiology • RA results in more than 9 million physician visits and more than 250,000 hospitalizations per year. Disability from RA causes major economic loss and can have a profound impact on families. RA affects 1% of the adult population this is due to low diagnosis (difficult to diagnose in early stages). • Females more than male. Pathophysiology Pathophysiology • The cause of RA is still unknown, it could be infection & as the case of other autoimmune diseases the immune system (AB to organism) begins to attack its self (molecules in the synovium that looks like the offending organism) Some infectious organisms mentioned in this context have been Mycoplasma, Erysipelothrix, parvovirus B19 and rubella, EBV, human herpes virus. Pathophysiology • Genetic predisposition is important, risk factors include cigarette smoking, hormonal factors (more in women). • Once triggered, B lymphocytes produce immunoglobins, & RF of the IgG & IgM classes that are deposited in the tissue, this subsequently leads to the activation of the serum complement cascade & recruitment of the phagocytic arm of the immune response, which further exacerbates the inflammation of the synovium, leading to edema, vasodilation and infiltration by activated T-cells Pathophysiology • Early and intermediate molecular mediators of inflammation include TNF-α, IL-1,6,8,15, transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. • Synovial macrophages and dendritic cells further function as antigen presenting cells by expressing MHC class II molecules, leading to an established local immune reaction in the tissue. Pathophysiology • The disease progresses in concert with formation of granulation tissue at the edges of the synovial lining (pannus) with extensive angiogenesis and production of enzymes that cause tissue damage. • Once the inflammatory reaction is established, the synovium thickens, the cartilage and the underlying bone begins to disintegrate and evidence of joint destruction accrues. S&S Synovitis • Most commonly affected joints are, small joints (including the hands, feet and cervical spine), but larger joints (shoulders, knees etc) can also be involved; the pattern of joint involvement can differ from patient to patient. • Inflammation in the joints manifests itself as a soft, "doughy" swelling, pain, tenderness to palpation and movement, local warmth, and functional impairment. • Morning stiffness is often a prominent feature and may last for more than an hour Deformity • The fingers are typically deviated towards the little finger (ulnar deviation) and can assume unnatural shapes. • Classical deformities are the Boutonniere deformity • swan neck deformity • The thumb may develop a "ZThumb" deformity Extra-articular • • • • • • • • general tiredness and lassitude, low-grade fever, elevated ESR, and anemia. GI bleeding as a side effect of drugs used in treatment, especially NSAIDs . Extra-articular manifestations occur in about 15% of patients Hepatosplenomegaly which may occur with concurrent leukopenia and is then referred to as Felty's syndrome,. lymphocytic infiltration affecting the salivary &lacrimal glands (Sjögren's syndrome). pericarditis, pleurisy, alveolitis, scleritis, and subcutaneous nodules. Management 1-Diagnosis Diagnostic Criteria The ACR has defined the following criteria (>4) for the classification of RA: • Morning stiffness of >1 hour most mornings for at least 6 weeks. • Arthritis and soft-tissue swelling of >3 of 14 joints/joint groups, present for at least 6 weeks • Arthritis of hand joints, present for at least 6 weeks • Symmetric arthritis, present for at least 6 weeks • Subcutaneous nodules in specific places • Rheumatoid factor at a level above the 95th percentile • Radiological changes suggestive of joint erosion Blood Test • RF (patients can be seronegative RA), not very specific test, 80% -ve in the 1st yr. • anti-citrullinated protein antibodies (ACPA), more specific, can detect pt in early stages or even before occurrence of the disease. • ESR, CRP, CBC, RF, liver enzyme, ANA, Ferritin can reveal hemochromatosis. 2-Document baseline disease activity & damage 3-Prognosis Prognosis • Selection of the treatment regimen requires a assessment of prognosis. Poor prognosis is suggested by earlier age at disease onset, high titer of RF, elevated ESR, and swelling of 20 joints . • Extraarticular manifestations of RA may also indicate poor prognosis. Prognosis • Studies have shown that patients with active, polyarticular, RFpositive RA have a 70% probability of developing joint damage or erosions within 2 years of the onset of disease Assessment of Disease Activity 4-Initial therapy Goals of Management • RA are to preventor control joint damage by inducing complete remission. • Prevent loss of function. • Decrease pain. • Maximize the QOL. Complete Remission Is Absence of: • 1) symptoms of active inflammatory joint pain • 2) morning stiffness • 3) fatigue, • 4) synovitis on joint examination • 5) progression of radiographic damage on sequential radiographs • 6)elevation of ESR or CRP levels Patient education • It is a chronic disease. • Risks of joint damage and loss of • Reviewing the risks and benefits of existing treatment modalities. • Periods of rest, job modification, time off from work, changes in occupation, or termination of work may be necessary Patient education • • • • • • Surgery Longitudinal treatment plan Treatment options Cost Adverse effects Expected time of response Non-Pharmacological Tx • • • • • • Patient education. Instruction in joint protection, conservation of energy, home program of joint range of motion strengthening exercises Regular participation in dynamic and even aerobic conditioning exercise programs Pharmacological Therapy General Guidelines • NSAIDs, glucocorticoid joint injection, and/or low-dose prednisone may be considered for control of symptoms. • The majority of patients with newly diagnosed RA should be started on DMARD therapy within 3 months of diagnosis. General Guidelines • DMARD control not cure so periodic assessment is required for efficacy & SE & therapy modification. • If disease activity is confined to one or a few joints, then local glucocorticoid injection may help. For patients with severe symptoms, systemic glucocorticoids may need to be initiated, or the dosage may need to be increased, for a short period of time. • Try to avoid narcotic addiction. Common DMARD General Guidelines • Pharmacologic therapy for RA often consists of combinations of NSAIDs, DMARDs, and/or glucocorticoids. NSAIDs • These agents have analgesic and antiinflammatory properties but do not alter the course of the disease or prevent joint destruction, so not used alone. • Choice of available agents is based on considerations of efficacy, safety, convenience, and cost. • Selective vr non-selective, no difference, cost. Risk factors for NSADs SE • advanced age. • history of ulcer. • concomitant use of corticosteroids or anticoagulants • higher dosage of NSAID • use of multiple NSAIDs • serious underlying disease Approaches to reduce SE • use of low-dose prednisone instead of an NSAID, • use of a nonacetylated salicylate, • use of a highly selective COX-2 inhibitor, • or use of a combination of an NSAID and a gastroprotective agent as: H2 blocker, PPI, PG analogue DMARDs • hydroxychlor oquine (HCQ), • sulfasalazine (SSZ), methotrexate (MTX), • leflunomide • etanercept • infliximab. • azathioprine (AZA) • D-penicillamine (D-Pen) • gold salts • minocycline • cyclosporine. Many factors influence the choice of DMARD • relative efficacy • Convenience of administration, • requirements of the monitoring program, • costs of the medication and monitoring • time until expected benefit • frequency • seriousness of adverse reactions. • Patient factors (compliance, diseases, prognosis, pregnancy/lactation). DMARDs • HCQ or SSZ first, • active disease or poor prognosis, MTX or combination therapy. • MTX alone or in combination should be included in patient with no MTX. • HCQ is indicated for milder disease, no monitoring (ophtha exam). • SSZ act more quickly, start gradually, watch for leucopoenia. DMARDs • Most select MTX as initial therapy esp. in severely active disease, CI: liver disease, renal impairment, lung disease, or alcohol abuse, monitor liver fx, contraception is recommended. • leflunomide as an alternative to MTX as monotherapy, especially for patients who cannot tolerate MTX, long t1/2, can be combined e MTX for max effect. DMARDs • AZA is rarely used • D-Pen is effective but its use is limited, in part, by an inconvenient dosing schedule. DMARDs • IM gold treatment is effective but injections are required every week for 22 weeks before less-frequent maintenance dosing is initiated, oral gold need 6 m to be effective. • Tetracyclines (minocycline), may be effective, needs more research. • Cyclosporine is beneficial as monotherapy and has short-term efficacy similar to that of D-Pen. The use of cyclosporine, however, has been limited by its toxicity, HTN & renal toxicity. DMARDs • Staphylococcal protein A immunoadsorption. Extracorporeal • immunoadsorption of plasma against a • staphylococcal protein A column was reported to be efficacious in a portion of patients with severe refractory RA . • Given the difficulty and cost of administering weekly treatments for 12 weeks, the limited duration of the response, and the high frequency of side effects, this treatment should be considered only for patients with refractory RA in whom treatment with several DMARDs has failed. • Glucocorticoids. Low-dose oral glucocorticoids (10 mg of prednisone daily, or the equivalent) and local injections of glucocorticoids are highly effective for relieving symptoms in patients with active RA. • Discuss ADRs. • 1,500 mg of elemental calcium/day ,400–800 IU of vitamin D/day. • Hormone replacement therapy • bisphosphonates Biological Agents • Anti–tumor necrosis factor (antiTNF-alpha)- biological agents etanercept and infliximab are beneficial in combination with MTX, avoid in TB, watch for infx, high cost. • Adalimumab: new agent • IL-1 receptor antagonist: Anakinra, modest effect. Combination therapy • Cyclosporine plus MTX • MTX, HCQ, and SSZ • Plus lo dose CS Practice points • glucocorticoids can offer immediate short-term relief and can serve as anadjunct to existing DMARD therapy. • the impact of glucocorticoids on structural damage and their optimal role in the management of RA require further investigation. Practice points • MTX remains the standard of therapy for both early and established RA ??? • MTX is effective as both monotherapy and in combination therapy • a step-up approach has been successful in improving clinical response and slowing radiographic progression Practice points • TNF-a inhibitors produce prompt clinical response and can slow radiographic progression • infections, including opportunistic infections, should be watched for and, if found, treated aggressively in patients receiving TNF-a inhibitors Guidelines for monitoring drug therapy in RA • These guidelines are drawn from a synthesis of expert opinion, a survey of rheumatologists, published guidelines, and, whenever possible, data on toxicity. • Toxicity may range from mild to serious and from reversible to irreversible. • We define rare toxicities as those which occur in <1% of patients using the agent, uncommon in 1-10%, and common in >10%. Toxicities of drugs used in RA that require monitoring include: • • • • • • • Gastrointestinal (GI) bleeding. Hypertension. Hyperglycemia. Macular damage. Renal damage. Hepatotoxicity. Myelosuppression. Reduction in the incidence, severity, & unfavourable outcomes of these toxicities can be attempted by • 1) pretreatment assessment to identify patients with risk factors for toxicity. • 2) careful patient & physician education about safe dosage & S&S of toxicity • 3) appropriate monitoring with physician follow up & periodic lab studies. NSAIDs Toxicities • dyspepsia (common). • gastric or small bowel bleeding or ulceration (uncommon). • renal insufficiency, confusion, depression, rash, headache, and hepatic toxicity (rare). NSAIDs Toxicities • reversibly inhibit platelet function and prolong bleeding time. • Patients with prior aspirin hypersensitivity are also at risk for developing bronchial spasms (rare). • There appear to be few differences in the frequency of serious toxicities among the different NSAIDs NSAIDs Toxicities To avoid GI SE: • Take drug with food. • Misoprostol should be considered for patients who require NSAID treatment & are elderly or have a history of PUD, GI bleeding, or CVD. • Sucralfate, H2 blockers, & antacids to reduce dyspipsea. NSAIDs Toxicities Renal complications: • High-risk groups for renal toxicity include • the elderly, particularly those receiving diuretics. • patients with preexisting renal disease, CHF, cirrhosis, atherosclerotic heart disease, or any altered physiologic state in which renal blood flow is being maintained by compensatory vasodilatation. NSAIDs Toxicities To prevent renal toxicity in patients who are at risk: • NSAIDs should be started in modest doses & then carefully increased. • Patients should be instructed to report if signs of fluid retention evidenced by weight gain or edema develop, if they become ill & dehydrated, or if they are to begin treatment with diuretics or ACE). NSAIDs Toxicities Renal complications: • monitor SrCr in high-risk patients every wk for several wks after Tx is started. • The average time of drug exposure has been 6.6 m for NSAID-induced nephrotic syndrome and 15 days for allergic interstitial nephritis. NSAIDs Toxicities Liver toxicity: • may cause elevation of liver enzyme levels, but severe hepatotoxicity is rare. • There is no evidence that abnormal findings on LFT in the absence of clinical symptoms change the outcome or are associated with serious hepatotoxicity • Liver function should be monitored in patients who are treated with diclofenac or in those who have intrinsic liver disease or in whom it is suspected. Table 1 DMARDs Hydroxychloroquine (HCQ) • The major toxicity of antimalarial agents is retinal damage (rare), which can lead to visual impairment. • It has less toxicity to be monitored. • Goal for monitoring is to detect early reversible retinal toxicity. Hydroxychloroquine (HCQ) The major risk factor for retinal toxicity appears to be the combination of: • cumulative dose >800 gm. • age >70 years (increased prevalence of macular disease). • HCQ dosage of >6.0-6.5 mg/kg/d, in patients with abnormal hepatic or renal function. Hydroxychloroquine (HCQ) • Patient should report any visual symptoms. • Baseline evaluation < 40 is not required. • After 6 month of therapy should do evaluation then every 6-12 months. • Patients with abnormal RF or those who have received HCQ > 10 years require more frequent ophthalmologic evaluation. • Table 1. Sulfasalazine (SSZ) Hematologic toxicities of SSZ: • leukopenia (1-3%) • thrombocytopenia (rare). • hemolysis in patients with (G6PD) deficiency. • agranulocytosis (rare). • aplastic anemia (rare) Sulfasalazine (SSZ) • Leukopenia is most likely to occur in the first 6 m of TX, may occur later. • Early dosage reduction &/or cessation may reverse leukopenia. • Patients should be questioned about allergies to sulfa drugs & cautioned about oligospermia. Sulfasalazine (SSZ) • The main goal of monitoring is to detect hematological toxicities. • a baseline assessment of AST or ALT in patients with known or suspected liver disease. • Table 1 Methotrexate (MTX) • The most serious toxicities of MTX include hepatic fibrosis (rare) & cirrhosis (rare), pneumonitis (uncommon), & myelosuppression. Methotrexate (MTX) • Independent risk factors for the development of serious liver disease in patients with RA include: • age • duration of therapy, • obesity, • diabetes, • alcohol intake, • prior history of hepatitis B or C Methotrexate (MTX) • Prevention of hepatic fibrosis and cirrhosis includes: • avoidance of MTX in patients with liver disease risk factor. • In patients with suspected liver disease, a pretreatment liver biopsy should be obtained. • Avoid alcohol consumption while taking MTX. • Patients should report symptoms of jaundice or dark urine. • Liver biopsy is recommended for patients with liver function abnormalities that persist during treatment with, or following D/C of MTX. Methotrexate (MTX) • Risk factors for myelosuppression include the use of antifolate agents such as trimethoprim, the presence of folate deficiency, and renal insufficiency. • CBC & RF q4-8wks. • Review of a radiograph obtained within 1 year prior to the initiation of MTX therapy is recommended to determine if preexisting lung disease is present and to provide a baseline for future comparison • Use fa supplementation. • pregnancy should be avoided. Gold compounds • The major serious toxicitieshematologic, renal, and pulmonaryrare. • monitoring should be considered if protein excretion is >500 mg/24 hours. • Oral less toxic than parental. • Patients need to be educated about the need for frequent monitoring & for reporting of rash, mucositis, hematuria or bleeding, or any new illness while receiving gold. D-penicillamine (DP) • rash (common), stomatitis (common), dysgeusia or metallic taste (common), myelosuppression (especially thrombocytopenia) (rare), & proteinuria (rare). • renal failure (rare) and induction of autoimmune syndromes such as SLE. • Slowly increasing the dosage of DP by 125250-mg increments every 3 months up to 750 mg/d to decrease thrombocytopenia. • Patients taking DP should report any new symptoms. Azathioprine (AZA) • capable of inducing myelosuppression at dosages used to treat RA (1-2 mg/kg/day). • The rationale for monitoring is to decrease the incidence and severity of myelosuppression. • Risk factors include: concomitant allopurinol or ACE inhibitors & RF. • Prevention: reducing the dose to 1/4 with concomitant allopurinol, avoiding ACEI, & decreasing the dose in patients with renal insufficiency. Glucocorticoids • increased appetite, weight gain, fluid retention, acne, cushingoid facies, HTN, diabetes, atherosclerosis, glaucoma & cataract, osteoporosis, a vascular necrosis, increased susceptibility to infection, & impaired wound healing. Glucocorticoids Patient's risk factors for steroid AE: • family Hx of diabetes, • established HTN or diabetes, • preexisting cataract(s) or glaucoma, • low BMD, • Hx of osteoporotic fracture, or significant osteoporosis risk factors such as premature menopause. Glucocorticoids • Initial assessment may include measuring Wt & BP, • serum glucose and cholesterol levels, • in patients at high risk for osteoporosis, consideration of BMD measurement & supplementation with calcium & vit D. • Baseline eye examination in patients over the age of 65 or with a family history of glaucoma. • Table 1. Antirheumatic agents and teratogenicity, lactation, and fertility • The majority of RA patients are females and some are at a reproductive age. • Decisions regarding the use of medications in pregnancy require consideration of the risks & benefits to mother & fetus.