May 17, 2013 - Rawan Albadareen
advertisement

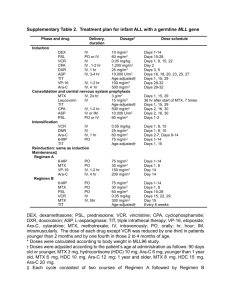
NEUROLOGY CASE PRESENTATION Rawan Albadareen, MD PGY-3 5/17/2013 CLINICAL PRESENTATION A 58 YO female with pre-B cell acute lymphoblastic leukemia (Philadelphia chromosome positive) w CNS involvement. She was undergoing CTX high dose MTX-Ara-C . Three days after last IT MTX, she complained of dysarthria and Lt sided weakness that fluctuated over the course of the day, started at 3 am that morning and by the time of evaluation around 3 pm was almost back to base line. Worth mentioning this had happened three times in the past and was associated with CTX. The first time this happened the episode was associated with confusion and picture of encephalopathy. Pt as well has tremors and distal numbness along with dryness of mouth and eyes PMHx, HTN HLP GERD ALL Hypothyroidism PSHx, Cholecystectomy FHx Non contributory SHx, Nonsmoker Nonalcoholic married PHYSICAL EXAM Mental status: alert, oriented to person/place/time Fluency Comprehension Articulation Repetition Naming Normal x x Abnormal x x x Normal II Pupils reactive, visual fields normal III, IV, VI EOMI, no nystagmus V Sensation nml V1-V3 Orbicularis Oculi: 5 VII Orbicularis Oris: 5 VIII nml to finger rub IX, X +strong cough XI Equal shoulder shrug XII Tongue midline Abnormal Trace facial droop on the Lt Motor: 5-/5 on the Lt side compared to 5/5 on the Rt (improved strength) Sensory; Intact to LT, glove and stocking distribution to pin prick Reflexes: Triceps Biceps Brachioradialis Patella Ankle Plantar Right 0 1 trace trace 0 equivocal Left 0 1 trace trace 0 equivocal Coordination: normal FTN, HTS, rapid alternating (resolved ataxia) Where What? ? DIAGNOSTIC STUDIES.. CSF cytology: -ve for malignant cells, 0 WBC/RBC, Normal Glucose/protein Infectious w/u: negative cultures and titers. MRI Brain 7/2/12 (at the time of initial diagnosis): Essentially unremarkable MRI BRAIN 12/6/12 Development of patchy and confluent areas of FLAIR hyperintinsity predominantly involving the deep bifrontal hemispheric white matter consistent with moderate nonspecific white matter disease. (TIME OF PRESENTATION) Leading diagnostic consideration include ADEM, chemotherapy induced necrotizing leukoencephalopathy or viral encephalitis. PML is an additional less likely consideration No acute or recent infarct. NEUROLOGICAL COMPLICATIONS OF IT MTX IT MTX is commonly associated with aseptic meningitis In addition, IT administration is rarely associated with: posterior reversible leukoencephalopathy syndrome, seizures subacute focal neurologic deficits lumbosacral polyradiculopathy noncardiogenic pulmonary edema pneumonitis and sudden death LEUKOENCEPHALOPATHY Leukoencephalopathy is a delayed complication of IT MTX, usually occurring after six months of therapy and when the cumulative IT dose of MTX exceeds 140 mg It is more likely in patients who receive concurrent whole brain radiotherapy or have previously received chemotherapy with intravenous MTX TRANSIENT LEUKOENCEPHALOPATHY AFTER IT MTX MIMICKING STROKE. Acute neurotoxicity with confusion, disorientation, seizures, and focal deficits may also be seen. This can clinically mimic stroke with restricted diffusion on MRI. However, unlike stroke, there is resolution of clinical and imaging findings within 1-4 weeks. Lesions exceeded the confines of adjacent vascular territories DWI findings seem to reflect cytotoxic edema within cerebral white matter suggesting a reversible metabolic derangement, rather than ischemia, as the basis for this syndrome. DWI CHANGES.. ADC MATCHING.. Stroke or not a Stroke… That is the question?? HYPERPERFUSION ON MRI IN ACUTE CHEMOTHERAPY-RELATED LEUKOENCEPHALOPATHY Magnetic resonance perfusion revealed mildly increased perfusion, a finding inconsistent with ischemic stroke. Magnetic resonance perfusion imaging proved valuable to rapidly distinguish acute chemotherapy-related leukoencephalopathy from ischemia. • El-Hakam LM, Ramocki MB, Riviello JJ, Illner A. Baylor College of Medicine IS IT PREVENTABLE? Prevention of neurotoxicity by high-dose folinic acid rescue after high-dose methotrexate and intrathecal methotrexate without compromising cure in spite of previous transient leukoencephalopathy after intrathecal methotrexate. Hamidah A, Raja Lope RJ, Abdul Latiff Z, Anuar ZM, Jamal R. • Ann Acad Med Singapore. 2009 Aug;38(8):743-4. CYTARABINE (ARA-C) High doses of Ara-C can cause an acute cerebellar syndrome in 10 to 25% of patients The pathogenesis of this syndrome is unknown, but there is widespread loss of Purkinje cells in the cerebellum. Symptoms range in severity from mild ataxia to an inability to sit or walk unassisted. Rarely, seizures develop. Less frequent complications peripheral neuropathies, brachial plexopathy, encephalopathy, lateral rectus palsy, optic neuropathy extrapyramidal syndrome THANK YOU!! MECHANISM OF ACTION Interference with folate metabolism Several key enzymes of these synthetic pathways are targets of MTX: Dihydrofolate reductase (DHFR) Thymidylate synthetase (TS) uses a methyl group from the reduced folate (dUMP) to (dTMP). MTX is considered an S-phase specific cytotoxic drug. The level of DHFR in any given cell is in great excess of what is needed to provide normal levels of reduced folates 95% DHFR needs to be blocked, reason behind the need for HDMTX IMPORTANCE OF POLYGLUTAMATION Increases the intracellular pool of folates, (not easily transported out of the cell because of their size and charge -continual cellular uptake of folates) The accumulation of PGMTX metabolites serves to further amplify and prolong the antiproliferative effects of MTX: Intracellular accumulation and decreased efflux of PGMTX enhances and prolongs inhibition of DHFR, since PGMTX is less readily dissociable from the enzyme than is free MTX Polyglutamated forms of MTX also bind to other enzymes involved in DNA synthesis such as TS, AICARFT, and GARFT; this further depletes intracellular thymidine and inhibits purine synthesis RATIONALE FOR LEUCOVORIN RESCUE MTX has little selectivity for tumor cells, and its effectiveness is limited by toxicity to normal tissue. Reduced folate to bypass the metabolic block induced by MTX within 24 to 36 hours. Folate transport is deficient in the malignant cells. In contrast to tumor cells, comparatively little PGMTX synthesis occurs in normal gut epithelium and bone marrow precursors under similar conditions RESISTANCE TO MTX Innate resistance (AML ;no polyglutamination) Acquired resistance: Decreased drug transport due to gene mutations or a change in the rate of transcription of the folate carrier Increased DHFR activity, typically due to gene amplification Mutations in the DHFR protein, which decrease its affinity for MTX Decreased cellular polyglutamation of MTX due to increased folyl polyglutamate hydrolase activity or decreased FPGS activity Decreased TS activity or affinity for the folate antagonists MTX is not typically used as a single agent for treatment of aggressive malignancy with some exceptions (primary CNS lymphoma, head and neck cancer, and malignant GTD).