Crohn*s Disease: Medical and Surgical Management
advertisement

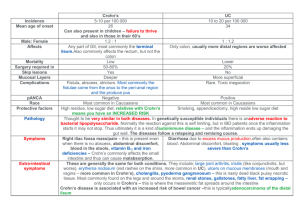
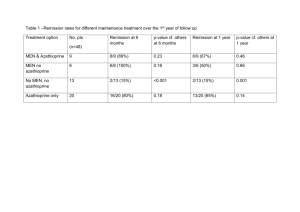
State of the Art Management of Crohn’s Disease Talal Al-Taweel Haya Al-Habeeb Gastroenterology Center Mubarak Al-Kabeer Hospital March 2015 S Disclosures S Travel/accommodation/meeting expenses: Janssen; AbbVie; Novartis Introduction S Definition Crohn's disease is a condition of chronic inflammation potentially involving any location of the alimentary tract from mouth to anus, but with a propensity for the distal small bowel and proximal large bowel. Inflammation is discontinuous along the longitudinal axis of the intestine and can involve all layers from mucosa to serosa History Intestinal inflammation characteristic of Crohn's disease first described by Morgagni in 1761 In 1932, the landmark publication of Crohn, Ginzburg, and Oppenheimer called attention to “terminal ileitis” as a distinct entity and chronic disease Underwent many naming changes due to discovery of multiple GI tract sites “Crohn’s disease” has been adopted to encompass the many clinical presentations of this pathologic entity General Approach S Step Up or Top Down? Step up: Begin treatment with drugs that have a relatively long track record and safety profile progressing to more potent (and potentially more toxic) treatments in patients with severe or refractory disease Top down: Aggressive treatment with more potent treatments (such as infliximab or other immunomodulators) relatively early in the course of disease, before patients become corticosteroid dependent and possibly even before they receive corticosteroids D’Haens et al. Lancet 2008 Weighing down the Value of TopDown Therapy Pros Early promotion of mucosal healing to prevent complications Evidence of immunomodulators and biologics to promote mucosal healing Cons Serious side effects Development of antibodies (biologics) Cost Majority of patients do not require more potent treatments initially So why not go all guns a blazing? Not every patient needs “top-down” or “early intensive therapy” We need to determine Who has high risk versus low risk disease How patients may respond to specific medications If patients agree with our plan Not everyone develops complications of Not everyone develops complications Crohn’s disease quickly of CD quickly 100 Cumulative Probability (%) 90 80 70 60 2 years 5 years Penetrating 50 40 30 Stricturing 20 10 Inflammatory 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 Months N= Cosnes et al. Inflamm Bowel Dis 2002 Who is going to have “bad” Crohn’s? Clinical Risk Factor Age of onset < 40 years Perianal lesion at diagnosis Required steroids for first flare Smokers P value 0.0004 0.01 0.0001 0.09 Beaugerie et al. Gastroenterology 2006 How about “good” Crohn’s? Cosnes et al. Gut 2012 Drugs S Sulfasalazine 5-ASA linked to sulfapyridine by an azo bond Salicylazosulfapyridine (sulfasalazine) was originally proposed as a treatment for rheumatoid arthritis Subsequently discovered that sulfasalazine was also efficacious in treating IBD. Sulfasalazine Large controlled clinical trials completed in the 1970s and the 1980s demonstrated benefits of sulfasalazine 3-6g/day over placebo for induction of remission with mild to moderate active ileocolonic and colonic CD Not effective for patients with active disease limited to the small intestine No consistent maintenance benefits after medical inductive therapy Use largely limited by side effect profile 5-ASA Salicylazosulfapyridine (sulfasalazine) was originally proposed as a treatment for rheumatoid arthritis Subsequently discovered that sulfasalazine was also efficacious in treating IBD. 5-aminosalicylic acid (5-ASA) medications were developed because many patients were intolerant of or allergic to sulfasalazine 5-ASA Conflicting older data of efficacy in CD, with variable definitions of remission and response Meta-analysis of three large trials with mesalamine, 4 g daily, demonstrated a statistically significant (P = 0.04) but a non-clinically relevant difference (CDAI benefit of 18 points) compared with placebo Two more recent meta-analyses in 2010 and 2011 showed mesalamine not to be superior to placebo in induction or maintenance of remission Hanauer et al. Clin Gastroenterol Hepatol 2004 Ford et al Am J Gastroenterol 2011 Geary Inflamm Bowel Dis 2007 Antibiotics Widely used but no consistent evidence for luminal disease Metronidazole Not more effective than placebo for inducing remission in mild to moderate disease Post hoc subgroup analysis indicated that metronidazole might be effective in patients with colonic involvement The well-documented risk of peripheral neuropathy necessitates monitoring for symptoms or signs of paresthesias Ciprofloxacin, rifaximin and anti-TB drugs - inconsistent evidence of efficacy in luminal disease Sutherland et al. Gut 1991 Antibiotics Perianal disease Use well established in fistulizing peri-anal disease Abscesses require surgical drainage Non-suppurative perianal complications of CD typically respond to metronidazole alone or in combination with ciprofloxacin No placebo-controlled maintenance trials, but it appears that continuous therapy is necessary to prevent recurrent drainage Steroids 2 large major published RCTs show that corticosteroids are effective agents for induction of remission in patients with CD. The National Cooperative Crohn’s Disease Study (NCCDS) Prednisone 0.5– 0.75 mg/kg daily with tapering over 17 weeks 60% clinical remission rates compared with 30% in the placebo group Steroids European Cooperative Crohn’s Disease Study (ECCDS) Prednisolone 1 mg/kg with tapering over 18 weeks 83% clinical remission vs. 38% receiving placebo Preponderance of evidence that low-dose conventional steroids are ineffective for maintaining remissions in CD and therefore should not be used as long-term agents to prevent relapse of CD IV vs. PO steroids? Parenteral steroids are used for severe or fulminant CD unresponsive to PO steroids No studies directly addressing issue, but overwhelming anecdotal evidence supports its use Methylprednisolone (60mg/day) preferred over hydrocortisone due to its decreased mineralocorticoid effects Budesonide Budesonide is a corticosteroid with a high hepatic first pass metabolism Predominantly topical glucocorticoid activity resulting in fewer systemic side effects compared with conventional steroids Best combination of short-term efficacy and safety in a series of well-controlled randomized trials. Greenberg NEJM 1994 Budesonide Controlled-release oral budesonide formulations at a dose of 9mg/day have been demonstrated to be more effective than mesalamine 4g/day and similar efficacy to steroids for the treatment of disease in patients with mild– moderately active CD involving the distal ileum and/or right colon Reduces the time to relapse in ileal and/or right colonic disease, but does not provide significant maintenance benefits after 6 months Seow Expert Opinion on Drug Metabolism and Toxicology 2009 Tromm Gastroenterology 2011 Thiopurines Thiopurines should be considered for patients who require two or more corticosteroid courses within a calendar year steroid dependent steroid refractory postoperative prophylaxis of complex (fistulizing or extensive) CD Slow onset of action, with a response usually seen within 3-6 months. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease (Review) Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. Thiopurines – induction Rx S U M M A R Y O F F I N D I N G S F O R T H E M A I N C O M P A R I S O N [Explanation] Azathioprine (AZA) or 6-mercaptopurine (6-MP) versus placebo for induction of remission in Crohn’s disease Patient or population: Patients with active Crohn’s disease Settings: Outpatients Intervention: Azathioprine or 6-mercaptopurine versus placebo Outcomes Illustrative comparative risks* (95% CI) Relative effect (95% CI) No of Participants (studies) Quality of the evidence (GRADE) Assumed risk Corresponding risk Control AZA or 6-MP versus placebo 372 per 10001 458 per 1000 (361 to 577) RR 1.23 (0.97 to 1.55) 380 (5 studies) ⊕⊕⊕⃝ Moderate2 Clinical remissionor im- 359 per 10001 provement 452 per 1000 (352 to 582) RR 1.26 (0.98 to 1.62) 434 (8 studies) ⊕⊕⊕⃝ Moderate3 Fistula improvement or 286 per 10001 healing 572 per 1000 (192 to 1696) RR 2.00 (0.67 to 5.93) 18 (3 studies) ⊕⊕⃝ ⃝ Low4 457 per 10001 612 per 1000 (466 to 809) RR 1.34 (1.02 to 1.77) 143 (4 studies) ⊕⊕⊕⃝ Moderate5 Withdrawals due to ad- 53 per 10001 verse events 90 per 1000 (50 to 163) RR 1.70 (0.94 to 3.08) 510 (8 studies) ⊕⊕⊕⃝ Moderate6 Serious Adverse events 38 per 10001 98 per 1000 (35 to 271) RR 2.57 (0.92 to 7.13) 216 (2 studies) ⊕⊕⃝ ⃝ Low7 Clinical remission Steroid sparing effect Comments * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95%CI). CI: Confidence interval; RR: Risk ratio 3 Chande et al. Cochrane Database Syst Rev 2013 Outcome: 1 Maintenance of 1.1. remission (Azathioprine)1 Maintenance of remission, Outcome 1 Maintenance of remission (Azathioprine). Analysis Comparison Thiopurines - maintenance Rx Review: Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease Peto Odds Ratio Study or subgroup Treatmentof remission Control Comparison: 1 Maintenance n/N n/N Weight Peto,Fixed,95%CI Outcome: 1 Maintenance of remission (Azathioprine) Peto Odds Ratio Peto,Fixed,95%CI 1 Azathioprine dose 2.5 mg/kg/day Candy 1995 Study or subgroup Summers 1979 14/25 16/19 2/20 Treatment n/N 15/20 1 Azathioprine dose 2.5 mg/kg/day Subtotal (95% CI ) 44 Candy 1995 Peto Odds Ratio Control n/N 11.3 % Peto ] 7.12 [ 2.11, 23.99 7.1 % P1.73 eto,Fixed,95%CI [ 0.37, 8.05 ] 18.3 % 4.13 [ 1.59, 10.71 ] Weight Peto,Fixed,95%CI 40 Odds Ratio 14/25 2/20 11.3 % 7.12 [ 2.11, 23.99 ] 16/19 Heterogeneity: Chi2 =Summers 2.00, df1979 = 1 (P = 0.16); I2 =50% 15/20 7.1 % 1.73 [ 0.37, 8.05 ] 40 18.3 % 4.13 [ 1.59, 10.71 ] Total events: 30 (Treatment), 17 (Control) Test for overall effect: Z = 2.92 (P = CI) 0.0035) Subtotal (95% 44 Total 30 (Treatment), 17 (Control) 2 Azathioprine dose 2.0events: mg/kg/day 2 df = 1 (P = 0.16); I29/29 =50% D’Haens 2007 Heterogeneity: Chi = 2.00, 18/32 16.5 % 2.73 [ 1.00, 7.45 ] 8.8 % 3.17 [ 0.80, 12.54 ] Test for overall effect: Z = 2.92 (P = 0.0035) Lemann 2005 2 Azathioprine dose 2.0 mg/kg/day 38/40 D’Haens 2007 O’Donoghue 1978 13/23 Lemann 2005 Rosenberg 1975 Willoughby 1971 O’Donoghue 1978 9/29 8/27 38/40 7/10 4/5 4/10 7/10 110 Total events: 80 (Treatment), 59 (Control) Subtotal (95% CI) 4/5 16.5 % 2.73 [ 1.00, 7.45 ] 8.8 % 3.17 [ 0.80, 12.54 ] 13.4 % 36/43 5.6 % 13/23 Rosenberg 1975 Subtotal (95% CI ) Willoughby 1971 36/43 18/32 8/27 2/5 13.4 % 2.9 % 4/10 114 110 Heterogeneity: Chi2 = 0.15, df = 4 (P = 1.00); I2 =0.0% 5.6 % 2/5 47.2 % 2.9 % 114 47.2 % 2.95 [ 0.97, 9.00 ] 3.16 [ 0.57, 17.62 ] 2.95 [ 0.97, 9.00 ] 4.48 [ 0.41, 49.42 ] 3.16 [ 0.57, 17.62 ] 3.014.48 [ 1.66, 5.45 [ 0.41, 49.42 ] ] 3.01 [ 1.66, 5.45 ] Total events: 80 (Treatment), 59 (Control) Test for overall effect: Z = 3.64 (P Heterogeneity: Chi=2 =0.00027) 0.15, df = 4 (P = 1.00); I2 =0.0% 3 Azathioprine dose 1.0 Test formg/kg/day overall effect: Z = 3.64 (P = 0.00027) Summers 1979 3 Azathioprine dose 1.0 mg/kg/day 37/54 Summers 1979 Subtotal (95% CI ) Subtotal (95% CI) 54 65/101 37/54 54 Total events: 37 (Treatment), 65 (Control) 101 34.5 % 65/101 34.5 % 101 1.20 [ 0.60, 2.41 ] 1.20 [ 0.60, 2.41 ] 34.5 % 1.20 [ 0.60, 2.41 ] 100.0 %% 100.0 2.32 1.55,3.49 3.49 2.32 [[1.55, ] ] 34.5 % 1.20 [ 0.60, 2.41 ] Total events: 37 (Treatment), 65 (Control) Heterogeneity: not applicable Heterogeneity: not applicable Test for overall effect: 0.52 (P = 0.60) TestZfor=overall effect: Z = 0.52 (P = 0.60) Total (95% CITotal ) (95% CI ) 208 208 255 255 Total events: 147 Total events: 147 (Treatment), 141(Treatment), (Control) 141 (Control) 2 = 7.75, df = 27 (P = 0.36); I2 =10% 2 = 7.75, df =Chi Heterogeneity: ChiHeterogeneity: 7 (P = 0.36); I =10% Test for overall effect: Z = 4.05 (P = 0.000050) Test for overall effect: Z = 4.05 (P = 0.000050) Test for subgroup differences: Chi2 = 5.60, df = 2 (P = 0.06), I2 =64% Test for subgroup differences: Chi2 = 5.60, df = 2 (P = 0.06), I2 =64% 0.1 0.2 0.5 0.1 0.2 Favours 0.5 placebo 1 2 Favours placebo 1 2 5 10 5 10azathioprine Favours Favours azathioprine Prefontaine et al. Cochrane Database Syst Rev 2009 Thiopurines Dosage AZA 2-2.5 mg/kg/day 6MP 1-1.5 mg/kg/day Safety/tolerance issues nausea/malaise (switch agents) lymphoma risk (4/10,000) opportunistic infections (vaccinate) pancreatitis (idiosyncratic) Myelosuppression (minimized if TPMT and metabolites checked) Methotrexate Alternative for the patient who does not tolerate or is unresponsive to azathioprine or 6-MP May be used in preference to azathioprine or 6-MP in patients with troublesome CD-related arthropathy. The drug should be initiated IM at a dose of 25 mg per week and a response anticipated within 3 months Methotrexate Remission rates reported up to 65% for MTX vs. 39% for placebo at 40 weeks Long-term open-label survival analysis has shown parenteral (but not oral) methotrexate to be effective in maintenance of a methotrexate-induced remission in 47% of patients with CD at 48 months Safety issues hepatic fibrosis interstitial pneumonitis teratogenicity nausea Biologics S Anti-TNF Engineered Antibodies Chimeric monoclonal antibody Human recombinant antibody Mouse Humanized Fab’ fragment VL VH No Fc CH 1 PEG Human IgG1 IgG1 PEG Infliximab PEG = polyethylene glycol Adalimumab Certolizumab pegol N Engl J Med 1997;337:1029-35 Lancet 2002; 359: 1541–49 NEJM 2004;350:876-85 NEJM 2010;362: 1383-95 Adalimumab – induction Rx Hanauer et al. CLASSIC-I, Gastroenterology 2006 Adalimumab – maintenance Rx Colombel et al. CHARM, Gastroenterology 2007 Adalimumab – LOR to infliximab Sandborn et al. GAIN, Am J Gastroenterol 2004 Can I combo adalimumab? No published studies specifically addressing issue of combination therapy of adalimumab with immunomodulator Sub-group analyses of major trials showed a trend towards a beneficial effect of combo vs. monotherapy Generally felt to be a class effect among all anti-TNF agents, although some experts would only use combination therapy with infliximab Vedolizumab Monoclonal antibody that binds to integrin α4β7 (LPAM-1, lymphocyte Peyer's patch adhesion molecule 1) Blocking the α4β7 integrin results in gut-selective anti- inflammatory activity by way of interfering with lymphocyte trafficking IV administration q8 weeks after induction dosing Recently FDA and EMA approved for both CD and UC Induction Maintenance Sandborn et al. NEJM 2013 Ustekinumab Human monoclonal antibody directed against interleukin 12 and interleukin 23 FDA approved for plaque psoriasis and psoriatic arthropathy Off-label use for refractory CD who are unresponsive or lost response to anti-TNF Sandborn et al. CERTIFI, NEJM 2012 Ustekinumab – McGill experience Kopylov, Al-Taweel et al. J Crohns Colitis 2014 CD Treatment Pyramid Predicted disease activity Very aggressive/ Refractory to Rx Surgery Ustekinumab Vedolizumab IMM + TNF antagonist Moderately aggressive/ More difficult to treat Uncomplicated/ easily treated AZA/6-MP/MTX Get it right the first time! Start the correct treatment at dx! Systemic corticosteroids TNF antagonists ( early intervention?) Budesonide Systemic corticosteroids SSZ (colonic dx) ? No Rx AZA, azathioprine; 6-MP, 6-mercaptopurine; MTX, methotrexate