Ni-Cu Complex Intro
advertisement
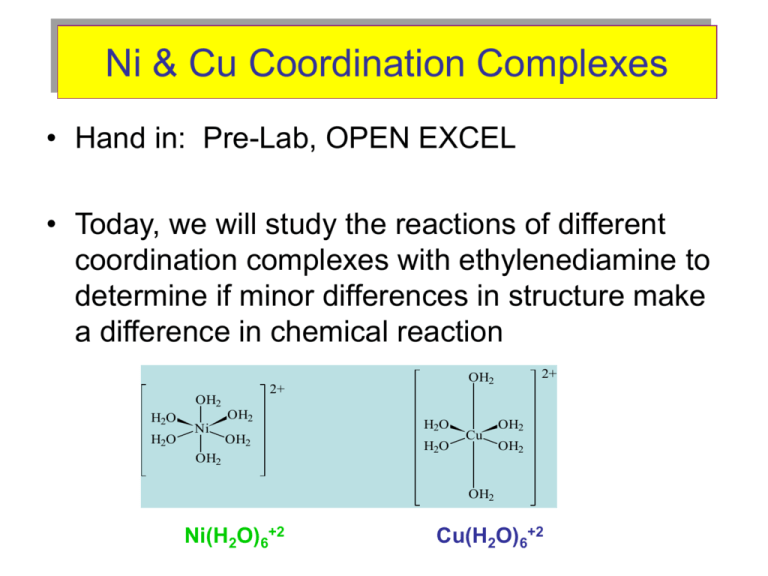
Ni & Cu Coordination Complexes • Hand in: Pre-Lab, OPEN EXCEL • Today, we will study the reactions of different coordination complexes with ethylenediamine to determine if minor differences in structure make a difference in chemical reaction 2+ OH2 H2O H2O OH2 Ni OH2 OH2 2+ OH2 H2O H2O Cu OH2 OH2 OH2 Ni(H2O)6+2 Cu(H2O)6+2 Ni & Cu Coordination Complexes Let’s consider the reaction between +2 ¨ CH CH ¨NH Ni(H2O) + H N 6 2 2 2 2 2+ OH2 H2O H2O Ni OH2 OH2 + N .. H2O .. H2NCH2CH2NH2 H2O OH2 Ni(H2O)6+2 N N Ni N N Ni 2+ N OH2 + 2H2O OH2 + ethylenediamine (en) Ni(en)3 +2 N coordination complex after N three additions of en N – N first step = H2NCH2CH2NH2 What will happen with Copper Complex’s slightly different geometry?? Ni & Cu Coordination Complexes For the Experiment: 1. Set up Data Collection and Graph in EXCEL, a. on the x axis: Time (in secs) collect data every 10 secs b. on the y axis: Temp (oC) – note temperature change, when temperature is stable and not decreasing, add next volume of (en) c. Graph Live so you know when to add the next (en) 2. Prepare Equipment a. Coffee Cup Calorimeter b. Thermometer from Drawer 3. Obtain Reagents – WRITE DOWN CONC. ON BOTTLES ALL VOLUMES MUST BE EXACT!!!! a. Trial 1 - Nickel Complex – GREEN b. Ethylenediamine (en) – IN FUME HOOD WEAR GLOVES DISPENSE INTO A TEST TUBE AND COVER WITH PAPER ADD EN UNTIL NO CHANGE IS NOTED IN TEMP. (No more than 4 steps for either Trial) c. Trial 2 – Copper Complex – BLUE Ni & Cu Coordination Complexes 4. Actual Experiment a. obtain baseline temperature data for at least one to two minutes b. add (en) c. immediately cover and gently swirl d. collect data until temperature plateaus e. try not to let temperature drop f. add next (en) to the solution and repeat until temp no longer rises (no more than 4 (en) additions per trial). 5. SAVE YOUR DATA, RENAME!!!, and go to trial 2 with Cu +2 complex 6. ALL WASTE IN LARGE CONTAINER IN FUME HOOD Ni & Cu Coordination Complexes – Graphing in EXCEL (page 4 step 3) Temperature (oC) Thermo-Profile of the Nickel Coordination Complex 25.8 25.6 25.4 25.2 25.0 24.8 24.6 24.4 24.2 24.0 23.8 23.6 23.4 23.2 23.0 22.8 22.6 22.4 22.2 22.0 21.8 21.6 21.4 21.2 0 50 100 150 200 250 300 350 400 450 500 550 Time (seconds) 38 . J 11 . g x qrxn x mL of each step x (T f Ti ) g o C mL H qrxn mol en 600 650 700 750 Ni & Cu Coordination Complexes – Calculations in EXCEL (Table on Page 5) Data Table to be generated in EXCEL by students: Reaction of Ni Ti Tf T V(soln) m(soln) S Step 1 3.8 Step 2 3.8 Step 3 3.8 qrxn (J) mol en H (kJ/mol) 0.00750 Average Reaction of Cu Ti Tf T V(soln) m(soln) S Step 1 3.8 Step 2 3.8 Step 3 3.8 qrxn (J) mol en Average H (kJ/mol) Ni & Cu Coordination Complexes What to do with the data? q rxn = - (3.8 J/g oC ) x masssol. x (Tf – Ti) Where: q rxn = energy released by the reaction mass sol. = TOTAL mass of liquid in the calorimeter Tf = Final Temperature in the STEP Ti = Initial Temp in the STEP So: Then: mass sol. = density of liquid (g/mL) x mL of TOTAL soln. H qrxn mol en Where: ΔH = Heat of reaction (kJoules/mole) mol (en) = moles of en in the STEP WATCH YOUR UNITS = Joules or Kilojoules!!!! Lab Report Title Page Purpose Results - Two Graphs (page 5, #1) Ni(H20)6+2 and ethylenediamine Cu(H20)6+2 and ethylenediamine - EXCEL Table, page 5, #2, (with sample calculations for Ni and Cu) - Present/Staple in order data/calculations were obtained/performed - Significant Figures and Units - Precision and Accuracy Conclusion Avg ΔH for Ni reaction and Avg ΔH for Cu reaction Number of en’s per Ni complex (question #3, page 5) Number of en’s per Cu complex (question #3, page 5) Errors and Improvements Individual Checked-Pre-labs








