Selection Rules for electronic transitions
advertisement

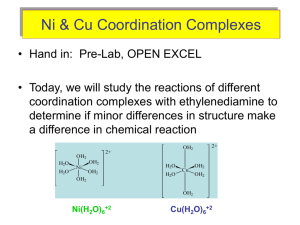
Chem 341 Coordination Chemistry – Electronic Spectra Chapter 11: pp 419-421, 428-429, 437-438 HW: 12 (assume o is lowest energy transition), 15, 16, 17, 18, 21, 24, 25, 27, Goals: Predict relative intensities of electronic transitions; distinguish dd, LMCT and MLCT; Distinguish excited states Upcoming: 11/18, 11/21: Ch. 11. Electronic transitions in metal complexes 11/23 no class 11/28, 11/30: Acc. Chem. Res. 2003, 36, 876-887 Photochemistry for solar energy 12/2: Exam III UMass Amherst Created by Karsten Theis Selection Rules for electronic transitions 1. Laporte selection 2. Spin selection How to relax these rules? • How to get rid of g symmetry? • Spin-orbit coupling ‘mixes’ spin states to relax rule 2 ... 2 ... 3 Why 2 peaks in the UV-Vis spectra of V(OH2)63+ A) the two electrons undergo transitions at different E due to ground-state Jahn-Teller distortion B) one is spin –allowed, the other is spin-forbidden C) ligand-to-metal charge transfers D) two different excited states (d-d) E) metal-to-ligand charge transfer ... 4 Why 2 peaks in the UV-Vis spectra of V(OH2)63+ 3T 1g 3T2g and 3T1g 3T1g Only 1e- moves at a time! Excited states have same spin multiplicity as ground state. Jahn-Teller distortion is weak here. Much stronger when eg* populated. ... 5 ... 6 Calculate CFSE for hexa-aquo(Cr3+) A) 7,000 cm-1 B) 17,000 cm-1 C) 24,000 cm-1 D) 50,000 cm-1 E) 650 nm What color is Cr(OH2)63+? ... 7 Calculate CFSE for hexa-aquo(Cr3+) D) 50,000 cm-1 What color is Cr(OH2)63+? Greenish Absorbs orange and violet ... 8 M(OH2)62+ Which peaks are spinforbidden? A) 1 only B) 2 only C) 1 and 2 D) 1, 2, 3 E) 4 1 3 2 4 9 ... M(OH2)62+ Which peaks are spinforbidden? C) 1 and 2 1 3 2 4 ... 10