Apr 30th
advertisement

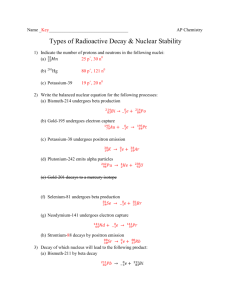
SI Chem 178 Ch 20, 21, and Review Leader: Emily Tuesday Apr 30, 2013 Review – Exam 2 16.6/16.7 Which is true: A large Ka value means the acid is weak OR A large pKa means the acid is weak. 16.10 H-X acid strength is affected by ___________ Oxyacid strength is affected by ______________ and ______________ How to remember this? Consider HF and HI… Consider HClO and HClO3 or HClO4… pH= How do we use Ka to calculate pH? (See p.671 to be walked through this) Write equations that relate Ka, Kb and Kw and pKa, pKb, pKw: Write out a titration curve for a weak acid being titrated against a strong base. ID the four areas and explain which equation must be used at each. (see p 718-719) Concepts: Ch 20/21 Electrolysis is spontaneous / non-spontaneous. What is the process of going from current and time to g of a substance? How do you convert between Coulombs and amps? Corrosion is spontaneous / non-spontaneous. How can corrosion be prevented? With this strategy in mind, what must you consider? When working nuclear problems involving EMISSION, the emitted particle ends up on the products OR reactants side. Alpha decay – Loss of: Beta decay- Loss of: Positron emission- Loss of: Or, conversion of: Or, conversion of: Gamma emission- Loss of: K-capture/e- capture: Or, conversion of: Problems 20.91 a. Cr3+(aq) is electrolyzed using a current of 7.60A. What mass of Cr(s) is plated after 2 days? b. What amperage is required to plate 0.250 mol Cr from Cr3+ solution over 8 hours? 20.93 Calculate the mass of Li formed by electrolysis of molten LiCl using a current of 7.5*10^4 A flowing for 24 hours. The cell is only 85% efficient though. What is the minimum voltage required to drive the reaction? (What is Ecell) (You must determine RedOx and cathode/anode.) 21.7a What are the numbers of protons and neutrons in 55 22 Mn? 21.11 Write balanced nuclear equations for the following: (See 21.12 for more practice!) a. Rubidium-90 undergoes B emission b. Selenium-72 goes through electron capture c. krypton-76 undergoes positron emission d. Radium-226 undergoes A emission 21.16 232/90 Th decays to 208/82Pb. How many alpha and beta emissions were required to complete this decay? 21.27 Complete and blance the following by supplying the missing particle: a. 252/98 Cf + 10/5 B 3 1/0 n + ? b. 2/1 H + 3/2 He 4/2 He + c. 1/1 H + 11/5B 3? d. 122/53 I 122/54Xe + ? e. 59/26 Fe 0/-1 e + ?