Measurements & The Metric System
advertisement

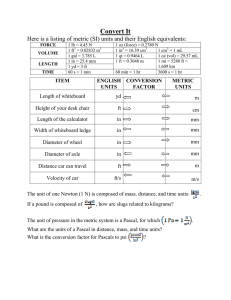
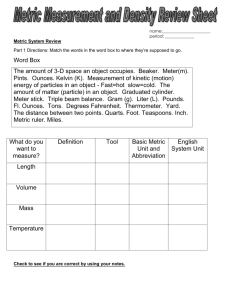
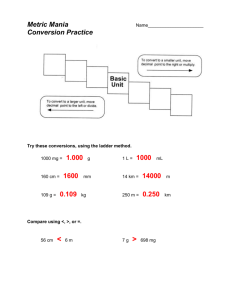
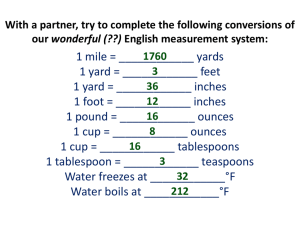
MEASUREMENTS, THE METRIC SYSTEM, AND DENSITY WHY DO WE USE THE METRIC SYSTEM? Almost all other countries are using the metric system Other countries’ companies are refusing to buy products from the U.S. if not labeled in metric units Scientists need a universal way to communicate data (SI Units) Lets look at our system… fractions of inch... 12 inches to a foot…. 3 feet to a yard…. 5.5 yards to a rod an... 320 rods to a mile... 43,560 sq. ft. to an acre... 16 ounces to a pound... 12 ounces to a pound... 4 quarts to a gallon... 3 teaspoons to a tablespoon… WHAT DOES THE METRIC SYSTEM MEASURE? The gram (g) measures mass A balance is used to measure mass. The liter (l) measures volume which is used when measuring liquids. A graduated cylinder is used to measure liquid volume. Length X Width X Height is used The meter (m) measures the length of an object or the distance from place to place. A ruler or meter stick is used to measure length. These are the base units of measurement. Prefixes plus base units make up the metric system. Staircase Conversions Metric units U.S. Customary Units Practice Problems 500 mL = ___ L 600 g = ___ kg 8 ounce carton of milk = ___ mL 218 pounds = _____ kg Density is defined as mass per unit volume. It is a measure of how tightly packed and how heavy the molecules are in an object. Density is the amount of matter within a certain volume. What is Density? If you take the same volume of different substances, then they will weigh different amounts. Wood Water Iron 1 cm3 1 cm3 1 cm3 0.50 g 1.00 g 8.00 g IRON Q) Which has the greatest mass and therefore the most dense? Density is the Mass per unit Volume DENSITY g/cm3 Aluminium 2.70 Iron 7.86 Brass 8.50 Wood Slate 0.50 2.80 Glass 2.50 Lead Marble Wax 11.3 2.70 0.89 Density Formula Density is mass divided by volume. Density = mass/volume The unit for mass is grams 3 The unit for volume is mL or cm The unit for density is… g/mL or g/ cm3 Units for density g/cm3 or g/ml Formula: M = mass V= volume D = density M=DxV V=M/D D=M/V Water and Density Since 1-gram of water has a volume of 1-mL then the density of water =1 gram/mL. 5o-mL of water will have a mass of 50 grams so again the density of water =1 g/mL. A kg of water will have a volume of 1000-mL so again the density of water =1 gram/mL. Floating Less dense materials will float on top of more dense materials. Objects with a density of less than 1-g/mL will float on top of water. Neutral Buoyancy Objects with a density equal to the density of water will float in mid water, at what ever level you place the object. Fish and submarines control their depth by changing their density. Objects that Sink Objects with a density greater than 1 g/mL will sink in water.