Day 4 Metrics - Day 1 Introduction to Chemistry and Measurement
advertisement

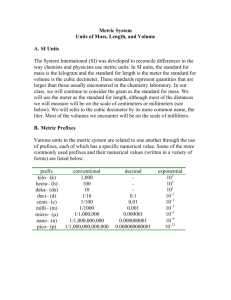
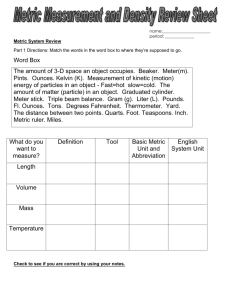
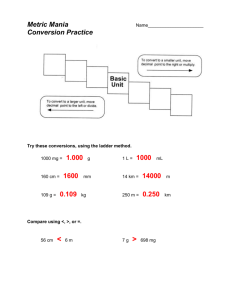
Appendix 1 - Measurements • We make QUALITATIVE observations of reactions — changes in color and physical state. • We also make QUANTITATIVE MEASUREMENTS, which involve numbers. –Use SI units —(International System of Units) based on the metric system Standards of Measurement When we measure, we use a measuring tool to compare some dimension of an object to a standard. For example, at one time the standard for length was the king’s foot. What are some problems with this standard? Some Tools for Measurement Which tool(s) would you use to measure: A. temperature B. volume C. time D. weight Learning Check Match L) length M) mass V) volume M A. ____ A bag of tomatoes is 4.6 kg. L B. ____ A person is 2.0 m tall. M C. ____ A medication contains 0.50 g Aspirin. V ____ D. A bottle contains 1.5 L of water. S.I. Measurement Based on: - number - units - accuracy report S.I. Units are: - related by factors of 10 * Table C of Reference Tables Metric Prefixes • Kilo- 10 3 3 or 1 kilometer = 10 m 1 km or 10 3 m • Centi- 10 -2 100 cm 1m 1000 m 1 km or 100 centimeters = 1 meter or 1m 100 cm • Milli- 10 -3 or 1000 mm 1m Metric Prefixes Base Units • Length = meter (m) • Mass = kilogram (kg) also gram (g) Mass vs. Weight Mass: Amount of Matter Weight: Force exerted by the mass, only present with gravity Temperature • Kelvin (K) • Measure of the average kinetic energy *Thermometer is a molecular speedometer. Shows vibrational energy. • Table T of Reference Tables Time The base unit for time = seconds (s) 60 seconds = 1 minute = 1/60 hour Amount of a Substance • Measured in moles (mol) • Contains Avogadro’s number of particles 23 • 6.02 X 10 • 602,000,000,000,000,000,000,000 Derived Units - Volume • • • • Liter (L) Volume = L X W X H 3 1cm X 1cm X 1cm = cm or 1ml 1dm X 1dm X 1dm = dm3 or 1L Derived Units – Heat Energy • Joules (J) • 4.18 J = the energy needed to raise the temperature of 1 gram of water 1 degree K. • Table B of Reference Tables – Specific Heat of Water Heat (q) = 4.18J/gK X g (water) X t Heat Energy • Suppose that a calorimeter contains 200. grams of water at 294.0 K. As an exothermic reaction occurs the water temperature rises to 314.0 K. • What is the quantity of heat transferred? 1. Temperature change of 20.0 K for 200g water. 2. q= 4.18 J/gK X 200g X 20.0 K 3. q= 16,720 J or 16.7 KJ 1 calorie = 4.18 Joules Pressure • Pascal (Pa) • Pa = force unit area • Table A of Reference Tables • 1 atm = 101.3 kPa Density • Table T of Reference Tables • Solve for m DXV=m • Solve for V = m D Density Practice 1. What is the density of an object with a mass 3 of 20.1g and a volume of 142.5cm ? 1. What is the mass of an object with a density of 32.1 g/cm3 and a volume of 10.0cm 3 ? 2. What is the volume of an object with a mass of 40g and a density of 10g/cm3 ?







![E3 8 ]DI C](http://s2.studylib.net/store/data/011239058_1-b2ecebb91eb7a6bba6f9583047b0aac1-300x300.png)