Metric System
advertisement

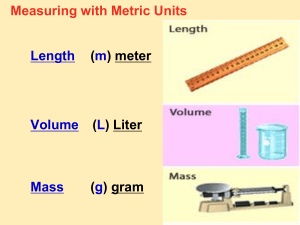
Metric System Scientific Measurements In Activity What country developed the metric system? What are the base units of the metric system? Distance base unit = Mass base unit = Volume base unit = In Activity What country developed the metric system? France What are the base units of the metric system? Distance base unit = meter Mass base unit = gram Volume base unit = liter In Continued Magic Equivalent Ruler Kilo Hecto Deka Base 1 g, m, l Deci Centi Milli Kings Have Diamonds But Diamonds Cost Money Metric System Developed by the French in the late 1700’s. Based on powers of ten, so it is very easy to use. Used by almost every country in the world, with the notable exception of the USA. Especially used by scientists. Abbreviated SI, which is French for Systeme International. Metric Prefixes Regardless of the unit (gram, meter or liter) the entire metric system uses the same prefixes. Common prefixes are: kilo = 1000 centi = 1/100th milli = 1/1000th 1 meter = 100 centimeters= 1000 millimeters Metric Prefixes Kilo Hecto Deka Base Units Deci Centi Milli 1000 base units 100 base units 10 base units Meter Liter Gram 10 of these make 1 base unit 100 of these make 1 base unit 1000 of these make 1 base unit Length Length is the distance between two points. The SI base unit for length is the meter. We use rulers or meter sticks to find the length of objects. Mass Mass is the amount of matter that makes up an object. A golf ball and a ping pong ball are the same size, but the golf ball has a lot more matter in it. So the golf ball will have more mass. The SI base unit for mass is the gram. A paper clip has a mass of about one gram. The mass of an object will not change unless we add or subtract matter. Measuring Mass We will use a triple beam balance scale and a digital top loading scale to measure mass. Your mass here on earth is the same as your mass on the moon. Mass is not the same as weight. Weight Weight is a measure of the force of gravity on an object. Your weight can change depending on the force of gravity. The gravity will change depending on the planet you are on. The SI unit for weight is the Newton (N). The English unit for weight is the pound. Gravity Gravity is the force of attraction between any two objects with mass. The force depends on two things, distance and mass. more distance = less gravity = less weight less distance = more gravity = more weight more mass = more gravity = more weight less mass = less gravity = less weight Weight and Mass Jill Earth 1 gravity Jupiter 2.5 gravities 30kg In orbit 0 gravity 30kg Moon 1/6th gravity 30kg mass weight 300N 50N 750N 0 Newtons 30kg Notice that Jill’s mass never changes. Her mother will not allow us to take parts off her, or add parts to her, so her mass stays the same. Jill is 30kg of little girl no matter where she goes! Stop Here! Day 1 Volume Volume is the amount of space contained in an object. We can find the volume of box shapes by the formula Volume = length x width x height In this case the units would be cubic centimeters (cm3). So a box 2 cm x 3 cm x 5cm would have a volume of 30 cm3 V = L x W x H Base Units The base unit for volume is the Liter. We measure volume with a graduated cylinder. Graduated Cylinders Liquids form curved, upper surfaces when poured into graduated cylinders To correctly read the volume, read the bottom of the curve called the meniscus Liquid Volume When the metric system was created, they decided that 1 cm3 of water would equal 1 milliliter of water and the 1 mL of water will have a mass of one gram. 1cm3 water =1 ml of water = 1 gram Water Displacement We can use water displacement to find the volume of objects that are not box shaped. We can put water in a graduated cylinder. If a rock causes the level to rise from 7 to 9 ml, the rock must have a volume of 2-mL. Water Mass and Volume 1 cm3 of water = 1 mL of water = 1 gram So what would be the mass of 50 mL of water? 50 grams So what would the mass of 1 liter of water be? 1 L = 1000 mL so its mass would be 1000 grams or a kilogram. Density Density is the amount of matter (mass) compared to the amount of space (volume) the object occupies. We will measure mass in grams and volume in ml or cm3 Density Formula Density is mass divided by volume. Density = mass/volume Remember, all fractions are division problems. Since the unit for mass is grams, and the unit for volume is ml or cm3, then the unit for density is g/ml, or g/ cm3 Density Formula Wheel Formula wheels make it easy to solve density problems. Cover the property you are trying to find, and do what is left over. To find density, cover the word density. You have mass over volume remaining. So divide mass by volume to find density! Mass density volume Density Formula Wheel To find mass, you cover the word mass. You now have mass times volume remaining. To find volume, cover volume. You have mass over density remaining, so divide mass by density to find volume. Mass density volume Understanding Density In the following illustrations, each will represent 1 cm3. Each g will represent 1 gram. Mass = 24g Volume = 8 cm3 g g g g g g g g 3 Density = 3g/cm g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g g •In other words, there are 3 grams in every cm3. Density Problem 2 g g g g g g g g g g g g •Mass = 12 grams •Volume = 6 cm3 •Density = 2 g/cm3 •In English we say the density of the object is 2 grams in every cubic centimeter. Density Problem 3 g g g g g g g g g g g ggg g g •Our previous problems were materials of uniform density. They were the same stuff throughout. But many materials are not. Gravel is a great example. We are finding the average density. •Mass = 16 grams •Volume = 8 mL •Density = 2 g/mL Water and Density Since 1-gram of water has a volume of 1-mL, then the density of water will always be 1 gram/ml. 5o-mL of water will have a mass of 50 grams, so again the density of pure water will be 1 g/ml. A kg of water will have a volume of 1000-mL, so it’s density will be 1 gram/ml. Floating and Sinking Less dense materials will float on top of more dense materials. Objects with a density of less than 1-g/mL will float on top of water. Objects with a density greater than 1 g/mL will sink in water. Neutral Buoyancy Objects with a density equal to the density of water will float in mid water, at what ever level you place the object. Fish and submarines control their depth by changing their density. Objects that Sink! Objects with a density greater than 1 g/mL will sink in water. Titanic Sails the Ocean Blue The Titanic is sailing on its maiden voyage. What is the density of this enormous, steel hulled ship, full of machinery, coal, people, and all sorts of heavy things? It’s floating, so it’s density must be less than 1 g/mL. How can this be? The Titanic is a hollow vessel full of air! Titanic verses Iceberg After HMS Titanic struck the iceberg, she started to fill with water. What happened to her density? As she took on more and more water, her density got closer and closer to 1 g/mL. The denser the ship became, the lower she settled into the water. Wreck of the Titanic What is the density of the Titanic resting on the ocean floor? Must be greater than 1 g/mL, as her steel hull is full of water instead of air. Comparing Densities •Where is the most dense object? •Where is the least dense object? Density Review We don’t actually count g’s to find the mass of objects. How would you find the mass of a rock? Use a balance scale. In real life, how would you find the volume of a rock? Use a graduated cylinder and see how much water the rock displaces. Out Practice Activity What is the mass of 50 ml of water _______? What is the volume of 50 ml of water _______? What is the density of 50 ml of water _______? What is the mass of the carriage bolt_______? What is the volume of the carriage bolt ______? What is the density of the carriage bolt ______?