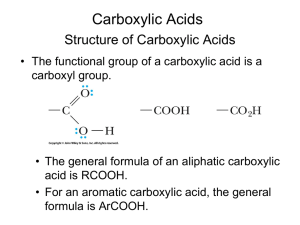carboxylate ion
advertisement

Ch 13 Carboxylic Acids, Esters, Amines and Amides SWBAT: Name/Draw compounds with functional groups Describe/Explain properties of functional groups Complete/Show reactions of functional groups Chapter 13.1 Carboxylic Acids Carboxylic Acids • Functional Group: Carboxyl Group – Carbonyl and Hydroxyl at the same carbon • Condensed writing: COOH • Carboxyl Carbon is C #1 • Alkyl prefix….end in -oic acid methanoic acid methylpropanoic acid 3-methylpentanoic acid Benzoic Acid • • • • Carboxyl on a benzene Carboxyl is attached to C #1 List all substituents by alpha Number either cw or ccw in order to get lowest numbers! 4-bromo-2-methylbenzoic acid Naming Practice • Parent alkane has to include carboxylic acid Drawing Practice Draw: 2-methyl-3-phenyl-pentanoic acid 4-iodo-2-isopropyl-benzoic acid Common Names Fatty Acids • Very long carboxylic acids • Which one has a higher boiling pt? Why? • Which one is a solid (fat) which a liquid (oil) at RT Alpha Hydroxy Acids (AHA) • Hydroxyl groups on the α Carbon: Carbon # 2 • Natural occurring acids in sour milk and fruits • Part of many skin care products to exfoliate and smooth skin Homework Chapter 13 Page 430 13.6 and 13.8 Chapter 13.2 Properties of Carboxylic Acids Acid Behavior • Acids are Hydrogen (H+) Donors • Carboxylic Acids dissociate in water and lower the pH aq COOH + H2O → COO- + H3O+ carboxylate ion & hydronium ion Boiling Point • Carboxylic Acids have the highest boiling point of organic molecules • More partial charges (4) than alcohols (3) Solubility • Carboxylic Acids dissolve very well in water • Form H-bonds with water molecules • But the longer the C-chain, the less soluble! Solubility Chapter 13.2 continued Reactions of carboxylic acids Dissociation Rx • When carboxylic acids dissociate they form a carboxylate ion Example: HCOOH → HCOO- + H+ Methanoic Acid Methanoate Ion Neutralization Rx • Carboxylate anions can react with metal cations to form carboxylic acid salts: COOH + NaOH → COO-Na+ + H2O Sodium methanoate Name the following: Potassium ethanoate or Potassium acetate Pentanoate Ion Homework Chapter 13 Page 434 13.10 – 13.16