Document
advertisement

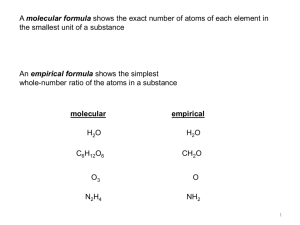
Unit 6 Chemistry Student Notes – Chemical Nomenclature Review: Label the periodic table with the number of valence electrons in each group and what charge their ion would have. Ag +1 Cd2+ Zn+2 Ionic compounds: Compound name Ions Criss Cross!! calcium chloride aluminum oxide lithium fluoride magnesium phosphide Formula CaCl2 Al2O3 Na2O BaI2 Compound name Final Formula Unit 6 Chemistry Student Notes – Chemical Nomenclature Ionic Compounds (with transition metals): Compound name Ions Criss Cross! Final Formula Iron (III) oxide Tin (IV) chloride Lead (IV) oxide Tin (II) sulfide Formula Non-Metal Ion Find Charge of Metal Name with Charge PbCl2 Cu2S SnO CoF3 Ionic Compounds (with polyatomic ions): Compound name Aluminum nitrate Ammonium phosphate Calcium hydroxide Copper (II) sulfate Zinc chlorate Ions Criss Cross! Final Formula Unit 6 Chemistry Student Notes – Chemical Nomenclature Covalent Molecules: Number of atoms Prefix 1 Do not use prefixes for IONIC compounds!! Only covalent!! 2 3 4 5 6 7 8 9 10 FORMULA NAME CO 2 N2O3 SO 3 NAME dinitrogen pentoxide chlorine monofluoride nitrogen trifluoride PREFIXES FORMULA Unit 6 Chemistry Student Notes – Chemical Nomenclature Acids: If anion ends in –IDE FORMULA If anion ends in –ATE ANION NAME If anion ends in -ITE ACID NAME HNO3 (aq) HF (aq) H3PO3 (aq) ACID NAME Nitrous acid Hydrobromic acid Acetic acid ANION NAME ANION ACID FORMULA-Add FORMULA H+ to anion Unit 6 Chemistry Student Notes – Chemical Nomenclature Seven Diatomic Elements I2, Br2, Cl2, F2, O2, N2, H2 You should know that the following elements exist as diatomic molecules when they are not bonded to another element. Remember the saying: I Bring Clay For Our New House Hydrogen H2 Oxygen O2 Nitrogen N2 Fluorine F2 Chlorine Cl2 Bromine Br2 Iodine I2 Balancing Chemical Equations: Unit 6 Chemistry Student Notes – Chemical Nomenclature Balance the following equations: Example 1: 1 Zn + 2@ HCl → Example 2: 1 Cu + 2 Example 3: 1 Al2(SO4)3 + Example 4: 1 Al4C3 + 1 AgNO3 → __ 3 STEPS: 1. 2. 3. 4. Practice: 1 H2O → 3 1 H2 2 Ag Al(OH)3 + 3 Cu(NO3)2 + Ca(OH)2 → Writing Balanced Chemical Equations: ZnCl2 + CH4 + 4 Al(OH)3 CaSO4 Unit 6 Chemistry Student Notes – Chemical Nomenclature Write and Balance the following equations: 1. A solid piece of Zinc reacts with Hydrochloric acid (HCl) to produce a solution of Zinc Chloride and Hydrogen gas as the products. 2. Sulfuric acid (H2SO4) is reacted with a solution of Sodium Hydroxide to produce a solution of Sodium Sulfate and water as the only products. 3. Chlorine gas is bubbled through a solution of Lithium Iodide, and the products are found to be a solution of Lithium Chloride and solid Iodine. 4. Solutions of Silver Nitrate and Barium Chloride are mixed, and the products are a precipitate of Silver Chloride and a solution of Barium Nitrate.