Crescent School - chemistry11crescentsummer
advertisement

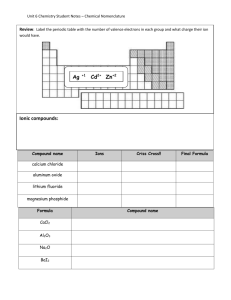
Crescent School SCH3U Overview: Chemical Nomenclature Simple Nomenclature can be divided into 3 categories: 1. Ionic compounds 2. Covalent (Molecular) Compounds 3. Simple Inorganic Acids See your text for practice problems and the power point lesson on our website. These will be outlined below: 1. Ionic Compounds cation(s) + anion(s) also includes polyatomic ions Ionic compounds do not exist as molecules. The ions exist in a 3-D crystal lattice. Think of the formula of an ionic compound as a “formula unit”—the lowest terms ratio of cation(s) : anion(s). don’t forget classical [eg. Fe2+ = ferrous; Fe3+ = ferric] and stock [eg. Fe2+ = Iron (II); Fe3+ = Iron (III)] naming systems for atoms that have more than one stable cation Combine cation(s) with anion(s) to obtain a neutral compound—Use “criss-cross” rule; reduce to lowest terms. DO NOT USE PREFIXES like mono, di tri, tetra, etc. with ionic compounds eg. 2. Binary Covalent (Molecular) Compounds made up of 2 non-metals names end in “–ide” use prefixes mono, di tri, tetra, penta, hexa, hepta, octa, nona, deca, etc can also use stock names where appropriate eg P2O5 eg. 3. Simple Inorganic Acids ACID: A substance that dissociates in water to produce 1 or more H+ ions. (We’ll refine this definition later.) Look for the physical state (aq) to indicate an acid. Three categories of acid names. Each depends on the name of the anion in the acid. 1. hydro_______ic acid — anion ends in “–ide”. eg. HCl(aq) = hydrochloric acid because the anion is chloride, Cl– eg Try: HBr(aq), hydrosulfuric acid, H2Se(aq), hydriodic acid, HF(aq), 2. ________ ic acid — anion ends in “–ate”. eg H2SO4(aq) = sulfuric acid because the anion is sulfate, SO42–. We need 2 H+ here to balance the 2– charge on the sulfate ion. (The “criss-cross” rule again.) eg Try: phosphoric acid, acetic (ethanioc acid), H2C2O4(aq), nitric acid, HClO3(aq), HClO4(aq) 3. ________ ous acid — anion ends in “–ite”. eg HNO2(aq) = nitrous acid because the anion is nitrite, NO2– eg Try: sulfurous acid = _____________, H3PO3(aq) = _____________________ —fin—