Unit 4
advertisement

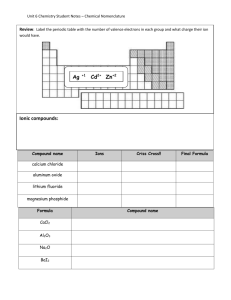
Unit 4 Nomenclature What’s Coming?? Nomenclature How we name stuff Common ions Nomenclature Common Names Sugar Epsom salts Gypsum Laughing gas TOO MANY CHEMICALS! The Naming System (IUPAC) Tells us about the composition Easy to construct the chemical formula Inorganic vs. Organic Making Ions Ion charges are based off of the valence electrons Metals LOSE electrons They become + Called cations Non-metals GAIN electrons They become – Called anions Electrons are lost or gained to obtain an octet Octet = 8 in the valence shell Transition metals are goofy Molecular Formulas The formula that indicates the specific ratio of the different atoms Tells us how many of each atom we need to have a specific compound Empirical Formulas ALL ABOUT THE RATIO: LOWEST common denominator Law of Definite Proportions A given compound ALWAYS displays EXACTLY the same ratio/proportion of elements H2O CO2 C6H12O6 Naming Binary Compounds Binary Compounds Composed of 2 elements Compounds contain either Metal and Non-metal – ionic compound 2 Non-metals Three types of binary compounds Representative Ionic – The metal forms only 1 type of cation and is a representative metal Transition Ionic– The metal forms more than 1 type of cation and is a transition metal Covalent– Two non-metals are joined together by a sharing of electrons Rules for Representative Ionic The cation is named first, the anion is named second A cation takes its name from the name of the element. An anion is named by taking the root of the element name and adding –ide to the end of the word. Representative Ionic Name the following compounds and indicate which ions and charges are present. Be sure to label each ion type. CsF AlCl3 MgI2 CaO KBr Rules for Transition Ionic Transition metals can have multiple charges…hence TRANSITION! Follow the same rules as Rep. Ionic but…. You must indicate the charge of the cation Use roman numerals and parentheses Fe+2 is iron (II)… Finding the cation charge can be difficult Find the anion charge first and balance using the given ratios Transition Ionic Provide the names for the following compounds. Be sure to indicate ion charge and to use both naming systems. CuCl2 HgO Fe2O3 MnO2 PbCl4 Rules for Covalent The first element in the formula is named first and the full element name is used. The second element is named as though it were an anion. Prefixes are used to denote the number of atoms present (see table). The prefix “mono” is never used for the first element, it is assumed. Latin Pre-fixes Mono = 1 Di = 2 Tri = 3 Tetra = 4 Penta = 5 Hexa = 6 Hepta = 7 Octa = 8 Covalent Name the following compounds. BF3 NO N2O5 Polyatomic Ions Polyatomic ions – ions made of 2 or more atoms These ions are assigned special names which must be looked up or memorized. I will provide you with a chart that you may use for your assessments. These ions are named using similar rules to ionic compounds. Polyatomic Ions Name the following compounds. Indicate the charge on each of the ions. Na2CO3 CsClO4 CuSO4 Rules for Naming Acids If the anion does not contain oxygen, the acid is named with the prefix “hydro” and the suffix “ic” is attached to the root name for the element. When the anion contains oxygen, the name is taken from polyatomic anion with a suffix of “ic” or “ous”. When the anion ends in “ate”, the suffix “ic” is used. When the anion ends in “ite”, the suffix “ous” is used. Acids Name the following acids. Indicate how many hydronium (H+) ions can be produced. HCl H2SO4 H2SO3 H3PO4 HF Nomenclature Etc. Quiz 1) BF3 2) HClO4 3) MgBr2 4) Co2O3 5) Nickel (III) Sulfide 6) Barium Phosphide 7) Phosphorus tetroxide 8) Manganese (IV) Phosphate Provide the #p+, #n0, valence e-, core e- for the following elements in the neutral state: 9) Si - 30 10) Se - 74 11) C - 14 12) W - 190