Electron Configuration Notes Sheet
advertisement
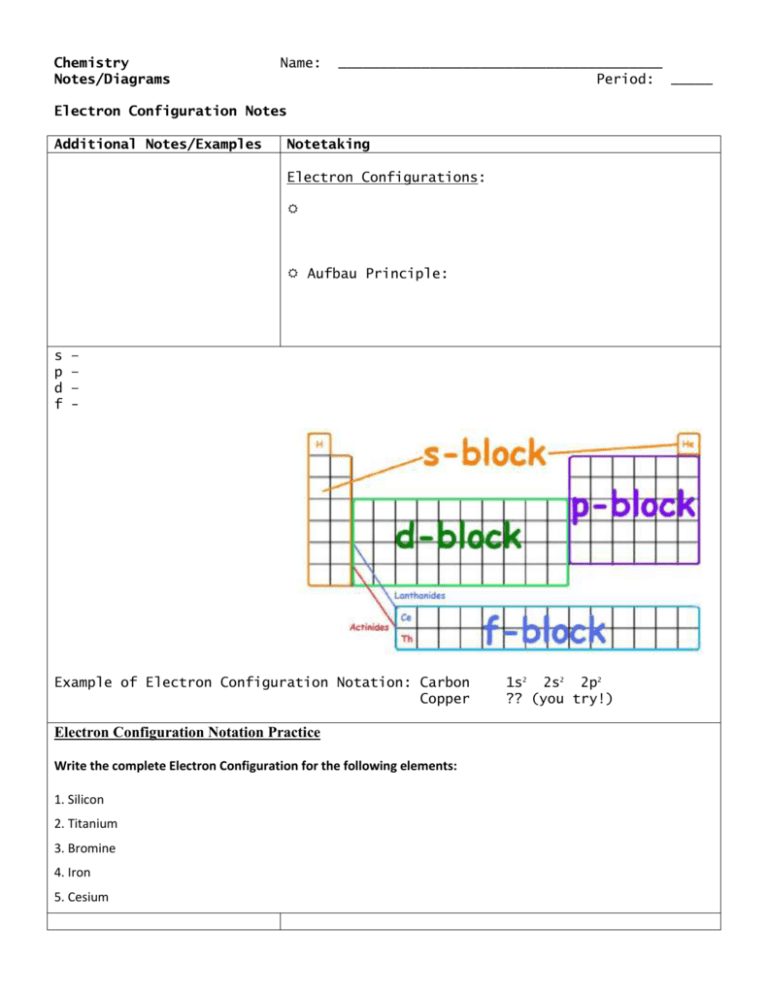
Chemistry Notes/Diagrams Name: _______________________________________ Period: _____ Electron Configuration Notes Additional Notes/Examples Notetaking Electron Configurations: Aufbau Principle: s p d f – – – - Example of Electron Configuration Notation: Carbon Copper Electron Configuration Notation Practice Write the complete Electron Configuration for the following elements: 1. Silicon 2. Titanium 3. Bromine 4. Iron 5. Cesium 1s2 2s2 2p2 ?? (you try!) Noble Gas Notation: Steps to writing noble gas notation: 1. 2. 3. 4. Examples: Magnesium Iron 1s2 2s2 2p6 3s2 [Ne] 3s2 1s2 2s2 2p6 3s2 3p6 4s2 3d6 [Ar] 4s2 3d6 Noble Gas Notation Practice Write the Noble Gas Notation for the following elements: 1. Phosphorus 6. Magnesium 2. Strontium 7. Potassium 3. Barium 8. Krypton 4. Bromine 9. Argon 5.Fluorine 10.Calcium Writing Orbital/Spin Diagrams: Based on: Pauli’s Exclusion Principle: Hund’s Rule: Examples of Orbital Diagrams and Electron Configurations: Ex: Carbon Ex: Chlorine 1s2 ___ 1s2 2s2 2p2 ___ ___ ___ ___ ___ 2s2 2p6 3s2 ___ ___ ___ ___ ___ Orbital Diagrams Practice: Complete the orbital diagrams for the following elements: Helium ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ Carbon ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ Magnesium ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ Potassium ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ Aluminum ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ Chlorine ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ ___ 3p5 ___ ___ ___ Additional Notes/Examples Notetaking Valence Electrons: Each element in a particular family has the same number of outer electrons, or __________ __________ Valence electrons determine the chemical characteristics of an element Determine the number of valence electrons for the following elements: 1. 2. 3. 4. 5. 6. Lithium Fluorine Magnesium Sulfur Potassium Nitrogen _____ _____ _____ _____ _____ _____ Lewis Dot Diagrams Shows: Valuable in showing how atoms share electrons in a covalent bond Steps: 1. The element symbol serving as the center of the diagram represents the nucleus and all inner level electrons except for the valence electrons in the outermost energy level. 2. Place electrons (dots/’x’) on all four sides of the symbol to represent up to eight valence electrons (depending on the element). 3. Remember to put one valence electron on each side before doubling up the electrons on any of the sides. Element Electron-Dot (Lewis-Dot) Structures Atomic Electron Electron-dot Number Configuration Structure Lithium Boron Carbon Oxygen Neon Chlorine Sulfur Magnesium Lewis Dot Diagram Practice: Write the Lewis Dot Diagram for the following elements: 1. Beryllium 4. Bromine 2. Nitrogen 5. Tin 3. Arsenic 6. Sodium Chemistry Electron Configurations Practice I WS Complete the following tables for each element identified. 1. Magnesium Atomic #: ___________ Orbital Diagram Electron Configuration Noble Gas Configuration # Valence Electrons Lewis Dot Structure 2. Bromine Orbital Diagram Electron Configuration Noble Gas Configuration # Valence Electrons Lewis Dot Structure Atomic #: ___________ Name: _______________________________ ____ period Chemistry Electron Configurations Practice II WS Name: _______________________________ ____ period Complete the following tables for each element identified. 1. Aluminum Atomic #: ___________ Orbital Diagram Electron Configuration Noble Gas Configuration # Valence Electrons Lewis Dot Structure 2. Germanium Orbital Diagram Electron Configuration Noble Gas Configuration # Valence Electrons Lewis Dot Structure Atomic #: ___________ Electron Configuration Practice and Review Directions: Read the instructions for each section below and answer in the space provided. Show all work for credit. Part I: Write the full electron configuration for the following elements. 1. Lithium: 2. Chlorine: 3. Silver: Part II: Write the noble gas electron configuration for the following elements. 4. Calcium: 5. Beryllium: 6. Arsenic: Part III: Draw the Lewis Dot structure for the following elements in the box provided. 7. Helium 8. Fluorine 9. Xenon 10. Silicon 11. Potassium Part IV: Identify the element from the following electron configurations. 12. 1s2 2s2 2p4 14. [Ar] 4s2 13. 1s2 2s2 2p6 3s2 3p5 15. [Kr] 5s2 4d9 Part V: Identify the highest energy level and how many electrons are in the highest energy level. 16. Lithium 18. Rhodium 17. Cesium 19. Xenon






