Honors Chemistry Reference Tables: Constants & Units
advertisement

NORTH SALEM: EXPANDED REFERENCE TABLES FOR HONORS CHEMISTRY.
BASED UPON THE 2011 NYS REFERENCE TABLES FOR CHEMISTRY
Table A: Standard Temperature and Pressure
Standard temperature 0.0 Celsius
(°C)
273 Kelvin
(K)
Standard pressure
1 atmosphere
(atm)
101.3 kiloPascals (kPa)
760 torr
760 mm Hg
Table B: Prefixes for Commonly Used Units
Prefix
Meaning
Interpretation (m)
piconanomicromillicentdecidekahectokilo-
10-12
10-9
10-6
10-3 one-thousandth
10-2 one-hundredth
10-1 one-tenth
10-1 ten
102 one hundred
103 one thousand
1 pm = 1 x 10-12 m
1 nm = 1 x 10-9 m
1 μm = 1 x 10-6 m
1 mm = 0.001 m
1 cm = 0.01 m
1 dm = 0.1 m
1 dam = 10 m
1 hm = 100 m
1 km = 1,000 m
http://physics.nist.gov/Pubs/SP811/sec06.html and
http://www.nist.gov/pml/wmd/pubs/upload/appc-13-hb44-final.pdf
A Few Common English to Metric Equalities
1 inch = 2.54 centimeters
1 pound = 454 grams
1 mile = 5,280 feet = 1.61 kilometers
1.06 quart = 1 dm3 or liter
1 calorie = 4.18 Joules
Table D: Selected Units
Symbol Name
m
g
Pa
K
mol
J
meter
gram
pascal
kelvin
mole
joule
s
min
h
d
y
dm3
M or M
second
minute
hour
day
year
cubic
decimeter
Liter
cubic meter
cubic
centimeter
parts per
million
parts per
billion
molarity
M
mole mass
μ
atomic mass
unit
L
m3
cm3
ppm
ppb
Table C: Constants for Water and Mole Theory
Water’s Melting / Freezing point
0.0 C
Water’s Normal boiling point
100 C
Water’s Specific Heat
4.18 J/g• K
Water’s Heat of Fusion constant
334 J/g
Water’s Heat of Vaporization constant 2,260 J/g
1.00 g/cm3
Water’s Density @ 3.98 C
Avogadro’s Constant
Molar Volume for gases @STP
Universal Gas Constant (R)
6.02 x 1023 species
22.4 L
0.08206 atmL/molK
Dimension or Quantity
length
mass
pressure
temperature
amount of substance
energy, work, quantity of
heat exchange
time
time
time
time
time
volume
volume (note 1 L = 1 dm3)
volume
volume
concentration
concentration
concentration
a mass in grams of
Avogadro’s constant of
species;
a gram formula mass (GFM)
atomic mass
Name
Table E: Selected Polyatomic Ions
Formula
Name
Ammonium
Formula
NH4+
Sulfite
SO32-
Hg22+
C2H3O2CH3COOCNCO32-
Sulfate
Thiosulfate
SO42S2O32-
Hydrogen Sulfate
Hypochlorite
Chlorite
Chlorate
Perchlorate
Chromate
Dichromate
Permanganate
Arsenate
Iodate
HSO4ClOClO2ClO3ClO4CrO42Cr2O72-
(not ammonia)
Mercury (I)
Acetate
Cyanide
Carbonate
Hydrogen Carbonate
Oxalate
Thiocyanate
Nitrite
Nitrate
Hydroxide
Peroxide
Phosphate
HCO3C2O42SCNNO2NO3OHO22PO43-
MnO4AsO43IO3-
•As a rule, use the –ate ending as a “starting point” or standard when multiple
versions of an ion exists. With this in mind, formulae & names have a pattern.
A suffix of –ITE means there is one fewer oxygen than the “standard”
Any ion with a name of HYPO____ITE has two fewer oxygen than the “standard”
Any ion named as PER _____ATE has one more oxygen, than that “standard”
•When no numeric charge is given, as in + or -, the value to be assumed is “1”
Table F: Solubility Guidelines for Ionic Compounds in Aqueous Solution
Ions That Form
Soluble Compounds
Group 1 ions
(Li+1, Na+1 etc…)
ammonium (NH4+)
Exceptions (thus insoluble)
none
Ions That Form
Insoluble Compounds*
carbonate (CO32-)
none
chromate (CrO42-)
nitrate (NO3-)
none
phosphate (PO43-)
acetate (C2H3O2- or
CH3COO-)
hydrogen carbonate
(HCO3-)
chlorate (ClO3-)
Grp 17 halides
(Cl-, Br-, I-)
sulfate (SO42-)
none
sulfide (S2-)
none
hydroxide (OH-)
none
when combined with
Ag+, Pb2+, Hg22+
when combined with
Ag+, Ca2+, Sr2+, Ba2+, or Pb2+
Exceptions (thus soluble)
when combined with Grp 1 ions
or ammonium ion (NH4+)
when combined with Grp 1 ions
Ca2+, Mg2+, or ammonium ion
(NH4+)
when combined with Grp 1 ions
or ammonium ion (NH4+)
when combined with Grp 1 ions
or ammonium ion (NH4+)
when combined with Grp 1 ions,
or Ca2+, Ba2+, Sr2+, or (NH4+)
*compounds having very low solubility (poor dissociation
into hydrated ions [electrolytes]) in water
Table G: Solubility Curves at Standard Pressure
Table H: Vapor Pressures of 4 Liquids
Vapor Pressure of Water at
Various Temperatures
(An Extension of Table H)
Temp Pressure Temp Pressure
(kPa)
(kPa)
(C)
(C)
0
0.6
25
3.2
3
0.8
26
3.4
5
0.9
27
3.6
8
1.1
28
3.8
10
1.2
29
4.0
12
1.4
30
4.2
14
1.6
32
4.8
15
1.7
35
5.6
16
1.8
37
6.3
18
2.1
40
7.4
19
2.2
50
12.3
20
2.3
60
19.9
21
2.5
70
31.2
22
2.6
80
47.3
23
2.8
90
70.1
24
3.0
100
101.3
Table I: Heats of Reaction at 101.3 kPa and 298 K
Reaction
∆H (kJ)*
CH4(g) + 2 O2(g) CO2(g) + 2 H2O(𝓁)
C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(𝓁)
2 C8H18((𝓁) + 25 O2(g) 16 CO2(g) + 18 H2O(𝓁)
2 CH3OH (𝓁) + 3 O2(g) 2 CO2(g) + 4 H2O(𝓁)
C2H5OH(𝓁) + 3 O(g) 2 CO2(g) + 3 H2O(𝓁)
C6H12O6(s) + 6 O(g) 6 CO2(g) + 6 H2O(𝓁)
2 CO(g) + O2(g) 2 CO2(g)
C(s) + O2(g) CO2(g)
4 Al(s) + 3 O2(g) 2 Al2O3(s)
N2(g) + O2(g) 2 NO(g)
N2(g) + 2 O2(g) 2 NO2(g)
2 H2(g) + O2(g) 2 H2O (g)
2 H2(g) + O2(g) 2 H2O (𝓁)
N2(g) + 3 H2(g) 2 NH3(g)
2 C(s) + 3 H2(g) C2H6(g)
2 C(s) + 2 H2(g) C2H4(g)
2 C(s) + H2(g) C2H2(g)
H2(s) + I2(g) 2 HI(g)
𝑤𝑎𝑡𝑒𝑟
KNO3(s) →
𝑤𝑎𝑡𝑒𝑟
NaOH(s) →
𝑤𝑎𝑡𝑒𝑟
NH4Cl(s) →
K+(aq) + NO3- (aq)
-890.4
-2219.2
-10943
-1452
-1367
-2804
-566.0
-393.5
-3351
+182.6
+66.4
-483.6
-571.6
-91.8
-84.0
+52.4
+227.4
+53.0
+34.89
Na+(aq) + OH- (aq)
-44.51
NH4+(aq) + Cl- (aq)
+14.78
𝑤𝑎𝑡𝑒𝑟
NH4NO3(s) →
𝑤𝑎𝑡𝑒𝑟
NaCl(s) →
NH4+(aq) + NO3- (aq)
Na+(aq) + Cl- (aq)
+25.69
+3.88
𝑤𝑎𝑡𝑒𝑟
-48.83
LiBr(s) →
Li+(aq) + Br- (aq)
-55.8
H+(aq) + OH- (aq) H2O(𝓁)
*The ∆H values are based on molar quantities represented in the
equations. A minus sign indicates an exothermic reaction.
Increasingly stronger oxidizing agent
E0 (V)
+2.87
+1.82
+1.50
+1.49
+1.44
+1.36
+1.33
+1.23
+1.07
+0.96
+0.90
+0.90
+0.85
+0.80
+0.77
+0.59
+0.54
+0.52
+0.49
+0.35
+0.34
+0.16
+0.15
0.00
-0.04
-0.13
-0.14
-0.23
-0.28
-0.40
-0.41
-0.74
-0.76
-0.83
-1.18
-1.66
-1.70
-2.38
-2.71
-2.76
-2.89
-2.90
-2.92
-3.04
Standard
Species to the right of the reaction arrow of the half
reaction are increasingly stronger reducing agents
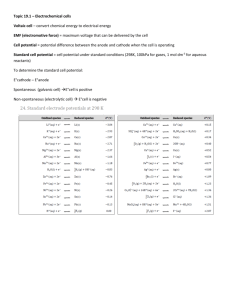
Table J: Table of Reduction Half-Reaction Potentials
F2(g) + 2e- → 2F-(aq)
Co3+(aq) + e- → Co2+(aq)
Au3+(aq) + 3 e- → Au(s)
MnO4-(aq) + 8H+(aq) + 5e- → Mn2+(aq) + 4H2O(l)
Ce4+(aq) + e- → Ce3+(aq)
Cl2(g) + 2e- → 2Cl-(aq)
Cr2O72-(aq) + 14H+(aq) + 6e- → 2Cr3+(aq) + 7H2O(l)
O2(g) + 4e- → 2O2Br2(l) + 2e- → 2Br-(aq)
NO3-(aq) + 4H+(aq) + 3e- → NO(g) + 2H2O(l)
2Hg2+(aq) + 2e- → Hg22+(aq)
ClO-(aq) + H2O(l) + 2e- → Cl-(aq) + 2OH-(aq)
Hg2+(aq) + 2e- → Hg(l)
Ag+(aq) + e- → Ag(s)
Fe3+(aq) + e- → Fe2+(aq)
ClO2-(aq) + H2O(l) + 2e- → ClO-(aq) + 2OH-(aq)
I2(s) + 2e- → 2I-(aq)
Cu+(aq) + e- → Cu(s)
IO-(aq) + H2O(l) + 2e- → I-(aq) + 2OH-(aq)
ClO3-(aq) + H2O(l) + 2e- → ClO2-(aq) + 2OH-(aq)
Cu2+(aq) + 2e- → Cu(s)
Cu2+(aq) + e- → Cu+(aq)
Sn4+(aq) + 2e- → Sn2+(aq)
2H+(aq) + 2e- → H2(g)
Fe3+(aq) + 3e- → Fe(s)
Pb2+(aq) + 2e- → Pb(s)
Sn2+(aq) + 2e- → Sn(s)
Ni2+(aq) + 2e- → Ni(s)
Co2+(aq) + 2e- → Co(s)
Cd2+(aq) + 2e- → Cd(s)
Fe2+(aq) + 2e- → Fe(s)
Cr3+(aq) + 3e- → Cr(s)
Zn2+(aq) + 2e- → Zn(s)
2H2O(l) + 2e- → H2(g) + 2OH-(aq)
Mn2+(aq) + 2e- → Mn(s)
Al3+(aq) + 3e- → Al(s)
Be2+(aq) + 2e- → Be(s)
Mg2+(aq) + 2e- → Mg(s)
Na+(aq) + e- → Na(s)
Ca2+(aq) + 2e- → Ca(s)
Sr2+(aq) + 2e- → Sr(s)
Ba2+(aq) + 2e- → Ba(s)
K+(aq) + e- → K(s)
Li+(aq) + e- → Li(s)
Table K: Formulae and Names of
Common Acids
Formula
Name
HCl(aq)
hydrochloric acid
HBr(aq)
hydrobromic acid
HI(aq)
hydroiodic acid
H2SO4(aq)
sulfuric acid
HClO4
perchloric acid
HNO3(aq)
nitric acid
HNO2(aq)
nitrous acid
H2SO3(aq)
sulfurous acid
H2PO4(aq)
phosphoric acid
H2S(aq)
hydrosulfuric acid
H2CO3(aq) or
carbonic acid
CO2(aq)
CH3COOH(aq) acetic acid (or more
or
formally, ethanoic
HC2H3O2(aq)
acid)
Table L: Formulae and Names of
Common Bases
Formula
Name
NaOH(aq)
sodium hydroxide
KOH(aq)
potassium hydroxide
Ca(OH)2(aq)
calcium hydroxide
NH4(OH)(aq) ammonium hydroxide (or
or NH3(aq)
aqueous ammonia)
Table M: Common Acid/Base Indicators
Acid / Base
Range / Color
Color Change
Indicator
In That Range
Methyl Orange
3.2-4.4
Red (below 3.2)
orange
Yellow (above 4.4)
Bromothymol Blue
6.0-7.6
Yellow (below 6.0)
(BTB)
green
Blue (above 7.6)
Phenolphthalein
8.2-10
Colorless (below 8.2)
(phth)
pink
Deep Pink (above 10)
Litmus
5.5-8.2
Red (below 5.5)
purple
Blue (above 8.2)
Bromcresol Green
3.8-5.4
Yellow (below 3.8)
green
Blue (above 5.4)
Thymol Blue
8.0-9.6
Yellow (below 8.0)
green
Blue (above 9.6)
Reference pH Scale & Indicators at 20°C
9
10
11
12
13
Drano
8
Tums
7
Baking Soda
6
BASE (alkaline)
Egg White
5
Physiological pH
4
NEUTRAL
Distilled Water
3
Tap Water
2
Black Coffee
1
Orange Juice
0
Vinegar (acetic acid)
ACID
14
Table N: Selected Radioisotopes
Nuclide Half-Life
Decay Mode
198
2.69 d
𝛽−
C
5730 y
𝛽
−
Ca
175 ms
𝛽+
5.26 y
𝛽
−
30.23 y
𝛽−
Au
14
37
60
Co
137
Cs
53
+
Nuclide Name
gold-198
carbon-14
calcium-37
cobalt-60
cesium-137
8.51 min
𝛽
Fr
27.5 s
francium-220
H
12.26 y
𝛽−
hydrogen-3
Fe
220
3
131
I
8.07 d
𝛽
37
K
1.23 s
𝛽+
potassium-37
42
K
12.4 h
𝛽
−
potassium-42
85
Kr
10.76 y
𝛽−
16
−
iron-53
−
iodine-131
krypton-85
N
7.2 s
𝛽
Ne
17.2 s
𝛽+
P
14.3 d
𝛽
−
Pu
2.44 x 104 y
plutonium-239
226
Ra
1600 y
radium-226
222
Rn
3.82 d
radon-222
90
Sr
28.1 y
𝛽−
strontium-90
19
32
239
99
Tc
232
Th
5
−
nitrogen-16
neon-19
phosphorus-32
2.13 x 10 y
𝛽
1.4 x 1010 y
thorium-232
technetium-99
233
1.62 x 10 y
uranium-233
235
7.1 x 108 y
uranium-235
uranium-238
U
U
238
U
5
9
4.51 x 10 y
ms = milliseconds, s = seconds, min = minutes
h = hours, d = days, y = years
Table O: Symbols Used In Nuclear Chemistry
alpha particle
beta particle
4
2He
or He-4 or
0
−1e
or
0
−1𝛽
4
2
𝛽−
gamma radiation
0
0𝛾
γ
neutron
1
0𝑛
n
proton
positron
1
1H
0
+1e
or
or
1
1p
p
0
+1𝛽
𝛽+
Table P Organic Prefixes
# of carbons
Prefix
1
meth2
eth3
prop(pronounced as in propeller)
4
but(pronounced as in beauty)
5
pent6
hex7
hept8
oct9
non10
dec12
dodec(a.ka. laur-yl)
16
Hexadec- (a.k.a. cet-yl or myrist-ic)
18
octadec(a.k.a. stear-yl)
20
eicos(a.k.a. arachid-ic)
Family
General Formula
Formula
alkane
CnH2n+2
alkene
alkyne
CnH2n
CnH2n-2
C4H10
C 4 H8
C 4 H6
Table Q: The Hydrocarbons
Examples
Name
Structure
butane
1-butene
1-butyne
Alternative Views
H H H H
|
|
| |
H—C—C—C—C—H
|
|
| |
H H H H
H H
| |
H—C—C—C C—H
| |
|
|
H H H
H
H H
| |
H—C—C—C C—H
| |
H H
CH3CH2CHCH
CH3 – CH2 – C ≡ CH
arene
(aromatic
hydrocarbon)
CnH2n-6
C 6 H6
benzene
note: the term
aromatic refers to
a closed ring with
C or N with
alternating
double bonds
where "n" equals the number of carbons in the longest (parent) chain
Table R: Organic Compounds With Functional Groups
Class
Description
a Example
Examples
a
Alcohol
R-OH
a
(Monohydroxy)
1 (O-H) group
bonded to a carbon.
Soluble in water
(polar molecule)
C3H7OH
b
C4H9OH
Alcohol
(Glycol or
Dihydroxy)
Aldehyde
Ester
2 O-H groups (or OH
groups) bonded to
carbon. Water soluble
(polar molecule)
O
||
R—C— H
A carbonyl group on
a terminal C with
carbon or hydrogen as
the "R" group
O
||
R—O—C—R'
a product of an
alcohol & carboxylic
acid reaction.
Ketone
b
(the most
common form
of organic acid)
Amine
(simple)
C3H6(OH)2
a
a
HCOH
b
CH3COH
a
CH3OOCCH3
b
C2H5OOCC2H5
O
||
R— C—R'
a carbonyl group on
an "interior" or nonterminal carbon
Carboxylic
Acid
C2H4(OH)2
O
||
R—C—O-H
a carboxyl group
(COOH) bonded to a
carbon R-Group.
RNH
|
H
a derivative of NH3
At least one H is
replaced with an
organic group. A
weak base (B-L)
a
C3H6O
b
C6H12O
(also called a
halocarbon)
R-X
Halogen(s) {X} are
substituted onto a
hydrocarbon, by
removing hydrogen(s)
1,2-ethanediol (ethylene glycol)
H H
| |
H—C—C—H
| |
OH OH
b
methanal (formaldehyde)
b
ethanal (acetaldehyde)
H O
| ||
H—C—C—H
|
H
CH3COOH
b
C2H5COOH
a
C3H7Br
b
C3H6F2
H H H
| |
|
H—C—C—C—H
| |
|
H O-H O-H
methyl ethanoate
H
O H
|
||
|
H—C—O—C—C—H
|
|
H
H
b
ethyl propanoate
H H
O H H
| |
|| |
|
H—C— C—O—C—C—C—H
| |
|
|
H H
H
H
a
2-propanone (acetone)
b
3-hexanone
H H H O H H
| | |
|| | |
H—C—C—C—C—C—C—H
| |
|
| |
H H H
H H
H O H
| || |
H—C—C—C—H
|
|
H
H
ethanoic acid (acetic acid)
H O
| ||
H—C—C—O—H
|
H
a
1-propanamine (1-propylamine)
b
1-bromopropane
Br H H
| |
|
H—C—C—C—H
| |
|
H H H
propanoic acid
H H O
| |
||
H—C—C—C—O—H
| |
H H
b
H H H
| |
|
HCCCNH
| |
| |
H H H H
C6H5NH2
a
1,2-propanediol (propylene glycol)
a
C3H7NH2
b
2-butanol
H H H H
| |
| |
H—C—C—C—C—H
| |
| |
H OH H H
O
||
H—C—H
a
Halide
1-propanol
a
a
Name / Structure
b
H H H
| |
|
H—C—C—C—O-H
| |
|
H H H
a
a
b Example
Name / Structure
phenylamine (aniline)
NH
|
H
b
1,2-difluoropropane
H H F
| |
|
H—C—C—C—H
| |
|
H F H
This page has been left blank, deliberately.
s block
1
18
Hydrogen
1.00794
1s1
Element Name
Sodium
Lithium
Beryllium
6.941
9.012182
p block (except for Helium)
22.989770
Relative Atomic Mass
2
Helium
Element Symbol
Atomic Number
13
[Ne] 3s1
Electron Configuration
14
15
16
4.002602
1s2
17
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Neon
10.811
12.0107
14.00674
15.9994
18.9984
20.1797
[He] 2s2 2p2
[He] 2s2 2p3
[He] 2s2 2p4
[He] 2s2 2p5
[He] 2s2 2p6
B
[He] 2s1
Sodium
22.989770
[Ne] 3s1
5
[He]2s22p1
[He] 2s2
Magnesium
[Ne] 3s2
3
4
5
6
7
Potassium
Calcium
Scandium
Titanium
Vanadium
Chromium
39.0983
40.078
44.955910
47.867
50.9415
51.996
54.938049
[Ar] 3d2 4s2
[Ar] 3d3 4s2
[Ar] 3d5 4s1
[Ar] 3d5 4s2
[Ar] 4s1
[Ar] 4s2
[Ar] 3d1 4s2
8
Manganese
*
9
10
11
Silicon
28.0855
[Ne] 3s2 3p1
[Ne] 3s2 3p2
14
12
Phosphorus
Aluminum
26.98153
d block
24.3050
30.973761
[Ne] 3s2 3p3
Iron
Cobalt
Nickel
Copper
Zinc
Gallium
Germanium
Arsenic
58.933200
58.6934
63.546
65.39
69.723
72.61
74.92160
[Ar] 3d7 4s2
*
[Ar] 3d10 4s1
[Ar] 3d8 4s2
[Ar] 3d10 4s2
[Ar] 3d10 4s24p1
Chlorine
Argon
35.4527
39.948
[Ne] 3s2 3p5
[Ne] 3s2 3p6
Si
55.845
[Ar] 3d6 4s2
Sulfur
32.066
[Ne] 3s2 3p4
[Ar]3d104s24p3
[Ar] 3d10 4s24p2
Selenium
Bromine
Krypton
78.96
79.904
83.80
[Ar] 3d10 4s24p4
[Ar]3d104s24p5 [Ar]3d10 4s2 4p6
2
Rubidium
Strontium
Yttrium
Zirconium
Niobium
Molybdenum
Technetium
Ruthenium
Rhodium
Palladium
Silver
Cadmium
Indium
Tin
85.4678
87.62
88.90585
91.224
92.90638
95.94
(98)
101.07
102.90550
106.42
107.8682
112.411
114.818
118.710
[Kr] 5s2
[Kr] 4d1 5s2
[Kr] 4d2 5s2
Cesium
Barium
Lanthanum
Hafnium
Tantalum
Tungsten
Rhenium
Osmium
Iridium
Platinum
Gold
Mercury
Thallium
Lead
Bismuth
Polonium
Astatine
Radon
132.90545
137.327
178.49
180.9479
183.84
186.207
190.23
192.217
195.078
196.96655
200.59
204.3833
207.2
208.98038
(209)
(210)
(222)
4f14 5d3 6s2
4f14 5d4 6s2
4f14 5d10 6s2 6p3
4f14 5d10 6s2 6p4
4f14 5d10 6s2 6p5
[Xe]4f145d106s26p6
[Kr] 5s1
[Xe] 6s1
Francium
(223)
[Rn] 7s1
[Xe] 6s2
138.9055
[Xe] 5d1 6s2
Radium
Actinium
(226)
(227)
[Rn] 7s2
*
[Kr] 4d4 5s1
4f14 5d2 6s2
Rutherfordium
(267)
Dubnium
(268)
*
[Kr] 4d5 5s1
*
4f14 5d5 6s2
Seaborgium
(271)
[Kr] 4d10 5s0
[Kr] 4d8 5s1
4f14 5d7 6s2
4f14 5d6 6s2
Bhorium
(272)
*
*
[Kr] 4d7 5s1
[Kr] 4d5 5s2
Hassium
(270)
4f14 5d9 6s1
Meitnerium
(276)
*
[Kr] 4d10 5s1
4f14 5d10 6s1
Darmstadtium
(281)
[Kr] 4d10 5s2
[Kr]4d105s25p1
4f14 5d10 6s2
Roentgenium
(280)
Antimony
[Kr]4d10 5s25p2
4f14 5d10 6s2 6p1 4f14 5d10 6s2 6p2
Copernicium
(285)
Tellurium
Iodine
Xenon
127.60
126.90447
131.29
121.760
[Kr]4d10 5s25p4
[Kr] 4d10 5s25p3
Flerovium
(289)
10
2
6
[Kr]4d10 5s25p5 [Kr]4d 5s 5p
Livermorium
(293)
[Rn] 6d1 7s2
Key:
indicates the existence of energy
levels equivalent to an atom of the
noble gas, [Xe], preceding the listed
sublevels for elements of atomic
numbers 58 - 85. This is used to
manage the available space.
italicized symbols indicate synthetic /
human made/ lab created elements,
although your notes will qualify this for
43Tc and 94Pu which were, at one time,
found naturally in the crust of the
Earth, but are now in miniscule
quantities & now must be synthesized.
( ) indicates the longest lived isotope of
an element for which the atomic mass
is indeterminate
*indicates that single atoms in the
gaseous phase of the element are
exceptions to the Aufbau Principle/
Madelung rule / Klechkowski rule
f block
Lanthanoid Series
Cerium
Praseodymium
Neodymium
Promethium
Samarium
Europium
Gadolinium
Terbium
Dysprosium
Holmium
Erbium
Thulium
Ytterbium
Lutetium
140.116
140.90765
144.24
(145)
150.36
151.964
157.25
158.92534
162.500
164.93032
167.259
168.93421
173.054
174.9668
4f1 5d1 6s2
4f3 6s2
4f4 6s2
4f5 6s2
Thorium
Protactinium
Uranium
Neptunium
232.038
231.03588
238.0289
(237)
2
2
[Rn] 6d 7s
[Rn]5f26d17s2
Actinoid series
[Rn]5f3 6d1 7s2
[Rn]5f4 6d1 7s2
4f 6 6s2
Plutonium
4f7 6s2
Americium
(244)
(243)
[Rn]5f6 7s2
[Rn]5f7 7s2
4f7 5d1 6s2
4f9 6s2
4f10 6s2
4f11 6s2
Curium
Berkelium
Californium
Einsteinium
(247)
(247)
(251)
(252)
[Rn]5f7 6d1 7s2
[Rn] 5f9 7s2
[Rn] 5f10 7s2
[Rn] 5f11 7s2
4f12 6s2
Fermium
(257)
[Rn] 5f12 7s2
4f13 6s2
Mendelevium
(258)
[Rn] 5f13 7s2
4f14 6s2
4f14 5d1 6s2
Nobelium
Lawrencium
(259)
(262)
[Rn] 5f14 7s2
[Rn]5f147s17p1
TABLE T: Selected Equations
Number of Nucleons
mass # = # of protons + # of neutrons
Relative Atomic Mass
Relative atomic mass = (%)(isotopic atomic mass) + (%)(isotopic atomic mass) + ...
Temperature
Density (g/mL or g/cm3)
K = C + 273
Density = mass
volume
Molar Density (g/mL)
Molar Density(g) @STP = M
22.4 L
where K = Kelvin Temperature & C = Celsius Temperature
where M = mole mass
Percent Composition
% composition by mass = mass of the Part you want x 100
mass of the Whole
Percent Error
% error = | measured value – accepted value | x 100
accepted value
Calorimetry
q = mc∆T
where q = energy in joules
m = mass in grams
c = specific heat in J/gram • K
∆T = change in temperature (may be in K or °C)
q = mHf
q = mHv
P1V1 = P2V2
T1
T2
where Hf = heat of fusion constant in J/g
Hv = heat of vaporization constant in J/g
where P = pressure
T = Kelvin Temperature
V = volume
PV = nRT
where P = pressure
T = Kelvin temperature
V = volume in Liters
n = number of moles
R = 0.08206 atmL/molK
m = mass in grams
M = mole mass
Combined Gas Law
Ideal Gas Law
PV = m RT
M
Moles (mol)
moles = mass in grams
M
where M = mole mass
Molarity (M or M or [ ])
Molarity = moles of dissolved solute
Liters of solution
Parts per Million (ppm)
Parts per Billion (ppb)
ppm = grams of solute x 106
grams of solution
%Mass/Mass
% m/m = mass of solute x 100
mass of solution
Titration (Neutralization)
(#H1+ )(Macid)(Vacid) = (Mbase )(Vbase)(#OH1-)
pH
pH
pH = -log[H3O1+]
pH + pOH = 14
ppb = grams of solute x 109
grams of solution
where V = volume
M = molarity
Table U: Summary of Selected Reactions
Reaction Type
Example
Comment(s)
Acid Base Neutralization
HNO3(aq) + KOH(aq) H2O(ℓ) + KCl(aq) + energy
Saponification
C51H88O6(ℓ) + 3 NaOH(aq) 3 CH3(CH2)14CO2Na(s) + CH2CHCH2(OH)3(aq)
an acid
in solution
a base
in solution
lipid
water
a base
in solution
+
a soap
CH4(g)
Combustion of a metal
2 Mg(s) + O2(g) 2 MgO(s) + energy
oxygen
a metal
Esterification
O2(g )
oxygen
CO2(g)
an alcohol
Na2O(s)
an ester in solution
water
carbonic acid in solution
+ H2O(ℓ)
water
water
associated with acid precipitation, soda or
seltzer water & blood pH
2 NaOH(aq)
sodium hydroxide (a base) in solution
a base in solution hydrogen gas
acid
in solution
a salt
carbon dioxide
a metal oxide in water produces a solution
with a basic (alkaline) pH
under some circumstances the H2 can
ignite in the O2 of air for a secondary &
explosive reaction
CaCO3(s) + 2 HCl(aq) CaCl2(aq) + CO2(g) + H2O(ℓ)
calcium carbonate
associated with flavorings / fats
water
a nonmetal oxide in water produces a
solution with an acidic pH
2 Na(s) + 2 H2O(ℓ) 2 NaOH(aq) + H2(g)
a metal
Metal Carbonate in Acid
water
+ H2O(ℓ) H2CO3(aq)
sodium oxide
Active Metal in Acid
burning in the presence of (di)oxygen gas
CO2(g) + H2O(ℓ) + energy
carbon dioxide
CH3COOH(aq) + CH3CH2OH(ℓ) CH3COOCH2CH3(aq) + H2O(l)
carbon dioxide
Metal Oxide in Water
glycerol
metal oxide
an organic acid
in solution
Nonmetal Oxide in Water
making of soap via the hydrolysis of a lipid
(a.k.a. glycerin)
Complete Combustion
(of an organic compound)
an organic
compound
linked to the controlled process of acid/base
titration
a salt
in solution
water
metal carbonates are weak bases, based
on Bronsted-Lowry Theory;
associated with the buffering of
lakes due to acid precipitation
Table V: Various Standard & Qualitative Tests
TEST FOR:
TEST
dioxygen gas O2(g)
Use a flaming splint
A POSITIVE TEST RESULTS IN, (PRODUCES, CAUSES)…
a flame to flare up and to burn more vigorously
dioxygen gas O2(g)
carbon dioxide gas
CO2(g)
Use a glowing splint
Bubble gas into Ca(OH)2(aq)
a glowing ember to re-light and to burn
a white precipitate (solid). Addition of more carbon dioxide reverses the
reaction, due to an acid / base reaction between excess carbonic acid (CO2 +
H2O) and the alkaline or basic Ca(OH)2(s) precipitate
hydrogen gas H2(g)
Use a flaming splint
metal (active)
React with a strong acid
water H2O(ℓ)
Use blue cobalt chloride paper
a popping sound (like uncorking a champagne bottle) because the dihydrogen
gas ignites in the presence of atmospheric dioxygen gas (O2(g))
bubbles filled with hydrogen gas. Often the un-reacted metal will darken in
color.
the blue color of a chemically treated paper to change to a pinkish/white color
Arrhenius acid
Test with various indicators
a color change in selected indicators (cross reference with Table M)
Arrhenius base
Test with various indicators
a color change in selected indicators (cross reference with Table M)
carbonate anion
React with an acid
fizzing due to bubbles of carbon dioxide gas
monosaccharides
(reducing sugar)
Perform Tollens’ test using
the precipitation/deposition of silver metal on glass
Ag(NH3)2+1(aq) + KOH(aq) + reducing sugar
Not all monosaccharides are reducing sugars. Fructose, is a structural isomer of
glucose, but fructose is not a reducing sugar.
monosaccharides
(reducing sugar)
Test with Benedict’s Solution
the color of the Benedict’s solution to change from blue to yellow or red due to
a reduction in the Cu2+ to Cu1+. Electrons are gained from the oxidized sugar
(reducing sugar). This test works well for many aldehydes and a few alphahydroxy-ketones [where a hydroxyl group is adjacent to the keto or carbonyl
group, called acyloins ]
polysaccharides (starch)
React with a solution of iodine
the color to change from red to blue/black by slightly altering the molecular
configurations of amylose (blue) and amylopectin (black)
protein
Use Biuret Test
a color change from blue to violet in the presence of proteins or blue to pink in
the presence of small polypeptides due to Co2+ forming coordination compounds
in basic solution
(metal hydroxide or ammonia H2O)