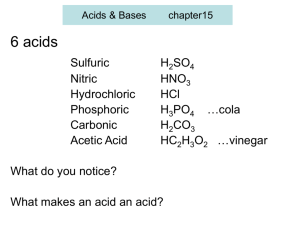
15 pH calculation part ONE

The student will be able to: calculate pH when given concentration of hydronimum ions / hydroxide ions / molarity
Ws 19.1
pH Scale pH measures [concentration] of H
3
0 + or OH pH ….. O – 14
0 3 7 10 14
Units of 10
4.6 pH is 10x stronger than 5.6pH
4.6 pH has 10x [H
3
0] than 5.6 pH
Pure water is neutral…..pH 7
“self-ionization of water
Water produces ½ H+ and ½ OH-
[H+]
[OH-]
pH = [H
3
0 + ] or [H + ] pH = power of the Hydrogen
Calculating pH from concentrations pH = -log [H
3
O + ] pOH = -log [OH ] pH + pOH = 14
Calculator
(-) log
number exponent )
enter
Find pH of a solution of hydronium concentration of [3 x 10 -5 ]?
pH = -log [3 x 10 -5 ]
(-) log 3 ×10 Carrott
^
-5 )
=
[1 x 10 -3 M] HCl
[1 x 10 -5 M] HNO
3
[0.001 M ] HCl
[0.09 M ] HBr
[1.34 x 10 -4 M] HCl
[7.98 x 10 -2 M] HNO
3
Calculate pH
A juice has a hydromium [1x10 -2 ]M.
What is its pH?
A juice has a hydromium [2.67 x 10 -2 ]M
What is its pH?
pH + pOH = 14
4
7 ph
2
6 pOH
8
13
=14
=14
=14
=14
=14
=14
Find the pH of a solution of Hydroxide concentration of [3 x 10 -5 ]
(-)log 3 ×10 -5 ) =
BUT it is hydroxide so…..it is the pOH….so subtract it from 14.
Calculate pH
[1 x 10 -4 M] NaOH
[1 x 10 -3 M] KOH
[1 x 10 -6 M] LiOH
[3.2 x 10 -2 M] Mg(OH)
2
[0.08 M] Ca(OH)
2
A juice has a hydroxide [1x10 -2 ].
What is its pH?
A juice has a hydroxide [3.24x10
-2 ].
What is its pH?
Calculate pH
Hydronium concentration of [.001M]
Hydroxide concentration of [.001 M]
H+ concentration of [ 1 x 10 -5 M]
OH concentration of [1 x 10 -5 M]
Molarity of HCl is [3.37 x 10 -3 ]
Molarity of KOH is [3.37 x 10 -3 ]
Ws.19.1
1.
A popular soda pop drink is measured to have a hydronium concentration of 1.34 x 10 -4 . What is the pH and is it acidic, base or neutral?
Ws.19.1
2.
The molarity of hydroxides in a pancake syrup is
1.0 x 10 -8 M. Pancake syrup by law must have a pH lower than 7 to be sold in the open market. Calculate the pH and identify if acid, base, neutral. Can it be sold?
3.
You work for Colgate Toothpaste. You need to make stannic fluoride solution of hydroxide molarity
[2.44 x 10 -4 ]. What is the pH? Is it acid, base, neutral?
4. In a sample of applesauce it is found that
[H
3
O]=2.51 x 10 -5 M. What is the corresponding pH?
Is the applesauce acid, base, or neutral?
5. The dead body’s blood has a H
3
O ion concentration of
[3.72 x 10 -8 M]. What is the pH of the blood? Is the blood acid, base, neutral?
6. What is the pH of one liter solution containing 0.56 grams of potassium hydroxide solute?
7. Which pH valve signifies the lowest hydroxide ion concentration?
a. pH3 b. pH 6 c. pH 7 d. pH 9 e. pH 13
8. What is the pH of a [1 x 10 -6 M] of KOH solution?
9. Which hydroxide ion concentration indicates the most base solution? a. 1 x 10-3 M b. 1 x 10-7 M c. 1 x 10-9 M d. 1 x 10-1 M
10. Calculate the pH of 0.09M solution of HBr acid.
11. Calculate the pH if the [OH]=3.22 x 10 -4 .
Is it acid, base, neutral?
Remember:
3 formulas: pH=-log[H
3
O] pOH=-log[OH] pH + pOH = 14
Remember:
[H
3
O] is hydronium concentration or…..molarity for an Acid or…..H+ proton concentration
Remember:
[OH] is hydroxide concentration or molarity for a base
The student will be able to: calculate ph when given concentration of hydronimum or hydroxide ions
Ws 19.1