Acid/Base Player/Coach Review
advertisement

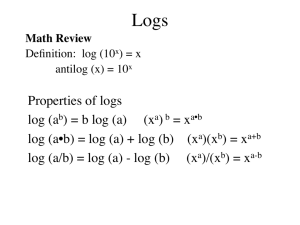
Player/Coach Acids and Bases- pH and Concentration A Player A 1. True or False: Every water solution contains both H3O+ (H+) and OH- ions. 2. In a water solution, [H3O+] x [OH-] = ??? 3. pH = -log ??? 4. A solution of HCl has a hydrogen ion concentration of 3.2 x 10-6 M. What is the pH of the solution? Would the solution be corrosive? 5. Another solution of NaOH has a hydroxide concentration of 3.2 x 10-2M. What is the pOH? What is the pH? Might this solution be corrosive? 6. What is the hydronium ion concentration of a solution of pH = 5.34? 7. What is the hydroxide concentration of a solution of pOH = 3.45? 8. What are the hydronium and hydroxide concentrations of a solution pH = 2.56? 9. A student titrates an unknown weak monoprotic acid solution with a 0.200 N NaOH solution. It requires 14.7 mL of the base to completely neutralize 10.0 of the acid. a. Write the general mathematical equation you will need to solve the problem. b. What is the concentration of the weak acid solution? Coach B Answers: 1. inversely 2. pOH 3. 14 4. pH = -log (0.256) = 0.59 Yes, probably very corrosive. 5. pOH = -log(0.000478) or –log(4.78 x 10-4) = 3.321 6. [H3O+] = antilog (0.80) = 100.80 = 6.3 M 7. [H3O+] = 1.5 x 10-6 M Probably not corrosive. 8. pH = 14 – 9.3 = 4.7 [H3O+] = 2.0 x 10-5 M [OH-] = 5.0 x 10-10 M 9. (0.100 N x 74 drops/ 40 drops = 0.185 N ammonia Player/Coach Acids and Bases- pH and Concentration B Player B 1. In a water solution, the hydronium and hydroxide concentrations are ___ proportional. (directly, inversely or not) 2. The negative log10 of the hydroxide concentration of a solution = ??? 3. pH + pOH = ??? 4. An acid solution has [H3O+] = 2.56 x 10-1 M. What is the pH? Would this solution be corrosive? 5. You are given a 0.000478 M concentration solution of KOH. What is the pOH of this solution? What is the pH? Is this a strong acid or base? 6. What is the hydronium ion concentration of sulfuric acid if its pH = -0.80? (Yes, it is possible to have pH values that are outside of the normal 0-14 range.) 7. A certain chemical dissolved in water has a pH of 5.82. What is the [H3O+]? Would this solution be corrosive? 8. What are the H3O+ and OH- concentrations of a solution of pOH = 9.3? 9. A student is trying to find the concentration of a sample of household ammonia. She finds that it takes 74 drops of 0.100 N HCl to titrates 40 drops of the ammonia. Assuming that the drop volumes for both liquids are equal, what is the concentration of the ammonia solution? Coach A Answers: 1. True 2. 1.0 x 10-14 M2 3. H3O+ 4. 5.49 5. pOH = -log(3.2 x 10-2) = 1.49 pH = 14 - 1.49 = 12.51 Yes 6. [H3O+] = antilog (-5.34) = 10-5.43 = 4.6 x 10-6 M 7. [OH-] = antilog(-3.45) = 10-3.45 = 3.5 x 10-4 M 8. [H3O+] = 2.7 x 10-3 M [OH-] = 3.6 x 10-12 M 9. a. NaVa = NbVb b. (0.200 N x 14.7 mL) / 10.0 mL = 0.294 N acid