Acid Base Chemistry Review Sheet
advertisement

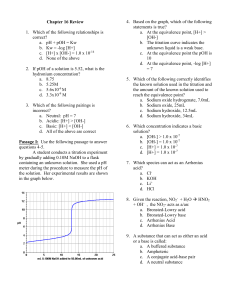
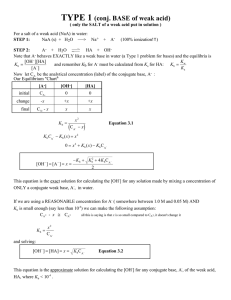
Acid Base test review Sheet 1. 2. 3. 4. List three properties of acids: _____________ _______________ ____________ List three properties of bases: _____________ _______________ ____________ Acids turn litmus ___________ and bases turn it ______________ Name the following acids: Put a check next to it if it is a strong acid HBr __________________ H2SO4 _________________ HClO4 ___________ HNO3 ________________ HI _____________________ H2S____________ HNO2 ________________ 6. What is the formula for carbonic acid? ______________ chloric acid ________ hydrochloric acid _______________________ 8. What is the formula for finding the pH of an acid? _______________ 9. What is the [H3O+] of a neutral solution? ____________ what is the [OH-] of a neutral solution? _______________ 10. What is the Kw of water? __________________ Define : 11. Arrhenius Acid _____________________________________________ 12. Arrhenius base______________________________________________ 13. Bronsted-Lowry acid ________________________________________ 14. Bronsted-Lowry base ________________________________________ 15. Lewis acid _________________________________________________ 16. Lewis base__________________________________________________ 17. indicator __________________________________________________ 18. titration ___________________________________________________ 19. Fill in the following table: [H+] pH [OH-] pOH Acid or base 10-11 5.6 2.27 x 10-5 8.33 20. The strongest acid would have a pH of ________, the strongest base would have a pH of ________ 21. What are the products of a neutralization reaction? 22. Write the balanced equation for the neutralization of Ca(OH)2 and HCl 23. Calculate the normality of an acid in which 7.73 grams of oxalic acid are dissolved in 375.0 ml of solution 24. How many grams of sulfuric acid would be required to produce a 500.0 ml of a .275 M solution? 25. How many ml of 0.40 M nitric acid are needed to neutralize 45 ml of 0.20 M NaOH? 26. What concentration of 30.0 mL of oxalic acid is needed to neutralize 25.0 ml of 0.30 M KOH 27. What molarity of 15.0 mL of sulfuric acid is needed to neutralize 17 ml of 2.3 M LiOH? 26. What is a titration used to determine? For the following equation: NH3 + H2 O <-> NH4+ + 27. What is the B-L acid _______________________ 28. What is the B-L base _______________________ 29. What is the conjugate acid ___________________ 30. What is the conjugate base? ____________________ H2SO4 + OH- <-> H2O + HSO4- 31. What is the B-L acid _______________________ 32. What is the B-L base _______________________ 33. What is the conjugate acid ___________________ 34. What is the conjugate base? ____________________ OH-