Slide 1
advertisement

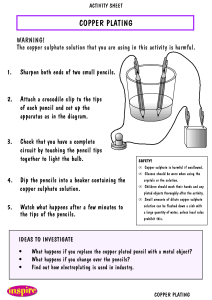
Starter question When 2g of copper carbonate (CuSO4) is added to 50cm3, 1 mol/l sulfuric acid (H2SO4), copper sulfate, carbon dioxide and water are formed. a) Write a balanced chemical equation for the reaction b) What mass of carbon dioxide is formed? c) If 1 mole of CO2 takes up 22.4l. What volume does this gas take up? Ionic Equations An ionic equation is used to tell us what is happening in a reaction To write an ionic equation there are a number of steps 1. Write a balanced chemical equation When copper sulphate is added to silver chloride, a precipitate of silver sulphate is formed along with copper chloride solution 2. Re- write the equation using ionic formulae Remember that covalent compounds and solids don’t get broken down into ions 3. Get rid of the spectator ions 4. Re- write the equation to show what is happening Today’s tasks •Complete Worksheet Homework Look up boyles Law. Write a paragraph explaining what it is. Remember to list your references