Carboxylic acids, esters and triglycerides
advertisement

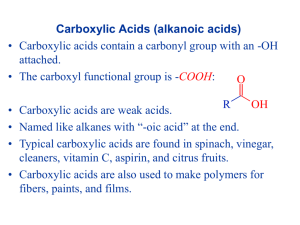
Carboxylic acids, esters and triglycerides L.O: Explain the water solubility of carboxylic acids Describe the reactions of carboxylic acids with metals, carbonates and bases Describe the esterification of carboxylic acids with alcohols in the presence of an acid catalyst, and also of acid anhydrides with alcohols Describe the hydrolysis of esters Describe a triglyceride as a triester of glycerol and fatty acids. Homework: carbonyl tests exam questions CARBOXYLIC ACIDS O O H -COOH or –CO2H NAMING CARBOXYLIC ACIDS Select the longest chain of C atoms containing the COOH group; • • Remove the e and add oic acid after the basic name • Side chain positions are based on the C in COOH being 1. Name this carboxylic acids: O H C OH H O H C C OH H HCOOH CH3COOH H H H O H C C C C OH H H H CH3CH3CH2COOH H H Br O H C C C C OH H H H CH3CH2CHBrCOOH Methanoic acid Ethanoic acid Butanoic acid 2-Bromobutanoic acid CH3 H H O H C C C C OH H H H CH3CH(CH3)CH2COOH 3-methylbutanoic acid In pairs discuss the solubility of acids. Clues: like dissolves in like, think about intermolecular forces between solvent and solute. O R C O R C O H + O Carboxylate ion Carboxylic acids are weak acids. The equilibrium is well over to the left. H+ In pairs: deduce the products of the reaction of carboxylic acids with: a) NaOH b) Carbonates c) Na CH3COOH(aq) + NaHCO3(aq) CH3COOH(aq) + NaOH(aq) 2 CH3COOH(aq) + Na2CO3(aq) CH3COONa (aq) + H2O (l) + CO2(g) CH3COONa(aq) + H2O 2 CH3COONa(aq) + H2O(l) + CO2(g) Esters • Made through the esterification reaction from a carboxylic acid and an alcohol Write out your own esterification reaction between methanol and methanoic acid using structural formula Now draw out the reaction between: 1. Ethanol and ethanoic acid 2. Propanoic acid and hexan-3-ol 3. 3,3-dichloro-2-methylhexanoic acid and butan-1-ol 4. Extension work: Name them Naming esters Draw these esters and name the alcohols and the carboxylic acids that made them: The smell of oranges is from the ester: 1. Octyl ethanoate Banana is made from: 2. Pentyl ethanoate Name these esters 1. Found in banana, pineapple, strawberry 2. Found in apple 3. Found in butter and cream 4. Making esters with acid anhydrides Hydrolysis of esters O R C O C2H5 O H+ catalyst + H2O + R C O H When an acid catalyst is used an equilibrium mixture of reactants and products is obtained CH2H5OH Base hydrolysis O R C O C2H5 O + + R C H2O CH2H5OH O H NaOH O R C O + Na + + H2O The salt of an acid is produced. This removes the acid from the reaction mixture so an equilibrium is not established. The reaction goes to completion . Uses of esters Uses of esters • Oil of wintergreen contains the ester methyl salicylate which is used to help relieve pains in mucles • Benzyl ethanoate is used to flavour foods to taste like apples and pears Fats and oils Fats and oils • Essential to maintain health • Solid fats = melting point above room temp, e.g. butter • Liquid oils = m.p below room temp, e.g. olive oil Esters of long-chain carboxylic acids. Name these two fatty acids • What does “saturated” and “unsaturated” mean? Quick questions 1. 2. 3. 4. Draw a molecule of glycerol What is the systematic name for glycerol? How does glycerol combine with fatty acids? What is the name for the bond? Abbreviating fatty acids