Neutralisation - Science @ St John's
advertisement

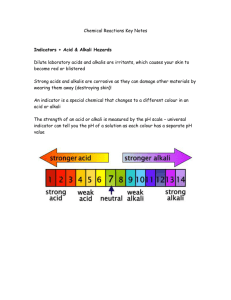
Title: Neutralisation What do you think happens when an acid and an alkali are mixed? ? Write an idea in your book! Stretch: People put vinegar on wasp stings. Looking at our title and what we were learning last lesson, suggest why!? The chemical reaction between an acid and an alkali is called neutralisation. acid alkali You need to remember this!! I’ll be testing you!! a salt water pH 7 LO: Describe a neutralisation reaction Level 4 Describe how to make a neutral solution Level 5 Describe a neutralisation reaction using a simple word equation Level 6 Explain a SPECIFIC neutralisation reaction using a word equation Make a mega fact mind-map on neutral solutions 7 Minutes What number is neutral on the pH scale? Stretch: Make these words into a neutralisation equation: Water Sodium Hydroxide Sodium chloride Hydrochloric acid +, +, Do you remember the word equation? What colour is a neutral solution with universal indicator? What is neutralisation? How do you achieve a neutral solution? Can you give an example of where neutralisation is used in everyday life. What number is neutral on the pH scale? pH7 Stretch: Hydrochloric acid + Sodium hydroxide Sodium Chloride + water How do you achieve a neutral solution? By adding an acid and an alkali together Do you already know a word equation? Acid + Alkali Salt + Water What colour is a neutral solution with universal indicator? GREEN What is neutralisation? Give an example of where neutralisation is used in everyday life. -Fertilizer -Bee and wasp stings -Antacid tablets Equipment Person 1 1. 10cm3 measuring cylinder 2. Test tube rack with test tubes of alkali Person 2 3. Pipette 4. Small beaker of HCl 5. Goggles I am looking for THE BEST neutral solution!! WHO CAN GET THE BEST GREEN? PUT GOGGLES ON 1. Add a couple of drops of universal indicator to the NaOH (Alkali) in the test tube 2. Use the pipette and carefully DROP BY DROP add the acid to the alkali. 4. Swirl the test tube as you do this 5. STOP when the solution goes green 6. Record how much acid it took to neutralise the alkali in your table. Level 6 Hard Stuff!! How do we know how to write word equations for SPECIFIC neutralisations? The Acid! The acid makes the last name of the salt! The Alkali The first part of the salt’s name is the same as the first part of the ALKALIS name. Hydrochloric Acid + Sodium Hydroxide Sodium Chloride + Water The salt! First part is the same as the alkali’s Second part comes from the acid! NEWS FLASH!! A LORRY CARRYING CONCENTRATED ACID HAS OVERTURNED AND BLOCKED THE ROAD- ACID IS EVERYWHERE! Write a news report for The Metro on this story!!! You must include: Level 4 - How could the fire service neutralise the acid - How could the Fire Service use indicators to show the strength of the acid? Level 5Explain how neutralising the acid works with an equation! EXTRAS: Why was the acid spill so dangerous? What hazard symbol might be on the lorry? Stretch: Hard word equations! Come and get a sheet and have a go for the scientific edition of the newspaper! Switch metro articles with the person next to you!! What level did they reach? Did they include? Level 4 - How could the fire service neutralise the acid - How could the Fire Service use indicators to show the strength of the acid? Level 5Explain how neutralising the acid works with an equation! EXTRAS: (Level 5+) Why was the acid spill so dangerous? What hazard symbol might be on the lorry? LO: Describe a neutralisation reaction Level 4 Describe how to make a neutral solution Level 5 Describe a neutralisation reaction using a simple word equation Level 6 Explain a SPECIFIC neutralisation reaction using a word equation Can you think of a keyword from today’s lesson? Write it on your post-it but DON’T LET ANYONE SEE Now stick it on the head of the person to your left. Now ask people on your table to describe the word to see if you can guess it!