Heating & Cooling Curves
advertisement

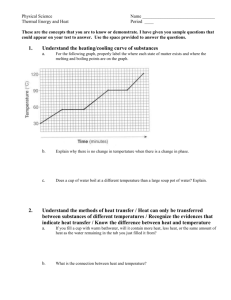
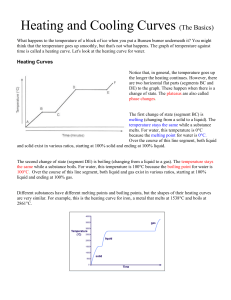
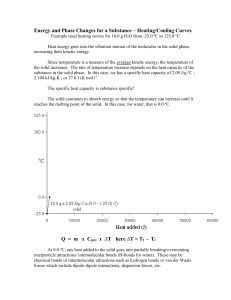
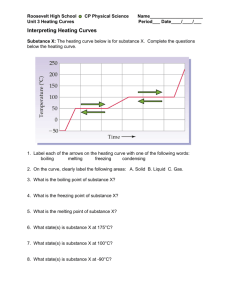
Warm-Up A 1.55 g sample of CH4O is burnt from 15C to 50C. If CH4O lost 725 J of heat what is the specific heat? Heating & Cooling Curves Heating & Cooling Curves To understand heating and cooling curves... Temperature The differences between solids, liquids and gases can be explained by the particle model: All substances are made up of particles These particles are attracted to each other, some strongly and others weakly These particles move around (i.e. have kinetic energy) The kinetic energy of particles increases with temperature Physical Changes Chemical Changes Physical Changes New substances are formed No new substances are formed Changes are usually permanent (irreversible) Changes are usually not permanent (reversible) States Of Matter Ice melting into water is an example of a physical change At a cold enough temperature, even substances that are normally gases will become solid At higher temperatures, solids change to become liquids or gases State Changes The Heating Curve of Water Heating Curves The two horizontal flat parts to the graph occur when there is a change of state The first change of state is melting - the temperature stays the same while a substance melts The second change of state is boiling - the temperature stays the same while a substance boils Cooling Curves Stearic acid cooling curves Cooling curves have horizontal flat parts where the state changes from gas to liquid, or from liquid to solid Change Of State boiling liquid gas melting condensing solid liquid freezing time Heating/Cooling WS Finish the post lab from Friday. On the WS: We have not discussed the heat of fusion or vaporization. Skip 18-20. We will come back to them later