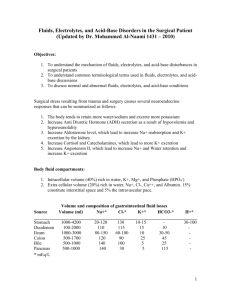
NURS 2140 Fluid and Electrolytes Acid Base and IV Therapy
advertisement

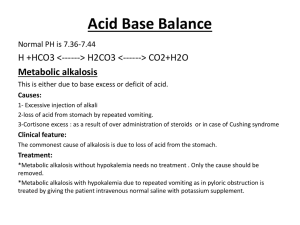
NURS 2140 Fluid and Electrolytes Acid Base and IV Therapy Teresa Champion, RN MSN Metropolitan Community College Winter 2012 BODY COMPOSITION AND FUNCTION • PRIMARY FLUID = Water – 60% total body weight (1 L = 2.2 lbs) – 2 – 2.5 L of water per day • TWO PRIMARY FLUID COMPARTMENTS – Intracellular (ICF) – Extracellular (ECF) • FUNCTIONS: – – – – – Transporting Removing Regulation Lubricating Food Digestion Intake = Output Regulation of Body Fluid • Osmosis – movement of water from lower particle concentration to higher particle concentration • Diffusion – movement of molecules from higher to lower concentration (simple or facilitated) • Filtration – movement of molecules through a semi-permeable membrane from higher concentration to lower concentration as a result of hydrostatic pressure There are two ways in which substances can enter or leave a cell: 1) Passive a) Simple Diffusion b) Facilitated Diffusion (carrier) c) Osmosis (water only) 2) Active a) Molecules b) Particles Oncotic vs Hydrostatic Pressure • Filtration is directly opposed by the oncotic pressure of plasma proteins, especially Albumin in the blood stream. • Arteriole – high hydrostatic pressure - (~32mmHg) • Venus – low hydrostatic pressure – (~15 mmHg) • Plasma oncotic pressure – (~22mmHg) • http://www.youtube.com/watch?v=VMvD29-Agtg • http://www.youtube.com/watch?v=mpg7ON2CfFE • http://www.youtube.com/watch?v=dAO8igIysaA • Homeostasis - thus in a steady state ECF = ICF OSMOLALITY • Number of molecules of solute per kg of water • NORMAL OSMOLALITY of blood is 275 – 295 milliosmoles per kg (mOsm/kg) of body weight – Isotonic Fluids - same osmolality as blood plasma) – Hypotonic Fluids - less concentration than blood plasma (< 275 mOsm/kg) – Hypertonic Fluids - greater concentration than blood plasma (>295 mOsm/kg) Homeostatic Mechanisms • Fluid Balance - regulated by: – Osmoreceptors of the hypothalamus - stimulates release of ADH and stimulates thirst. – Baroreceptors (pressure sensitive cells) in carotids and aorta also stimulate the release of ADH – Baroreceptors in glomerular arterioles in kidney will secrete Renin and start the Renin-Angiotension (RAA) cascade thus resulting in release of aldosterone from the adrenal glands and cause sodium retention = fluid retention (water follows sodium) Role of the Heart • Atrial Natriuretic Peptide: (ANP): secreted from atrial cells of heart (in response to too much volume in the blood) – acts as diuretic – inhibits thirst mechanism – suppresses the RAA cascade Role of the Kidneys • Filter approx 180 Liters of blood per day; GFR (glomerular filtration rate) • Produces urine between 1-2 Liters/day • If loss of 1% to 2% of body water, will conserve water by reabsorbing more water from filtrate; urine will be more concentrated • If gain of excess body water, will excrete more water from filtrate; urine will be more diluted Lab Tests for Evaluating Fluid Balance SERUM Normal Levels Osmolality 275-295 mOsm/kg of water Hematocrit 40-50%* BUN 5-20 mg/dl URINE Normal Levels Specific Gravity 1.005 – 1.030 Osmolality 50-1200 mOsm/kg of water Sodium 135 – 145 mEq/L Potassium 3.5 – 5.0 mEq/L Sodium 40-220 mEq/day Chloride 95-108 mEq/L Potassium 25-100 mEq/day Bicarbonate (CO2) 22-28 mEq/L -Arteriole 24-30 mEq/L -Venous Evaluation of Fluid Status • Normal serum hematocrit – 40 – 50% • Dilute serum – Low hematocrit and electrolyte levels • Concentrated serum – Elevated hematocrit and electrolyte levels Clinical Manifestations of Overhydration and Dehydration OVERHYDRATION DEHYDRATION Crackles in lungs Dry mouth and tongue, mucous membranes S3 Heart Sound Tachycardia Dyspnea Severe thirst (maybe not in elderly) Reduced blood oxygen levels and increased CO2 levels – respiratory acidosis Increased temperature (may rise 1 or 2 degrees) Bounding pulses Weak pulses Increased blood pressure Orthostatic Hypotension, systemic hypotension Increased edema, ascites “tenting” skin Increased neck swelling – jugular vein distension Flat neck veins Decreases in HCT, Serum Osmolality, Serum Sodium, Potassium, Chloride, Bicarbonate, BUN; Urine Specific Gravity < 1.005; Urine Osmolality >1,200 mOsm/kg Increases in HCT, Serum Osmolality, Serum Sodium, Potassium, Chloride, Bicarbonate, BUN, Creatinine; Urine specific Gravity >1.030; Urine Osmolality < 50 mOsm/kg Nursing Considerations • • • • • • • Assess for headache, dizziness, syncope CHF-SOB, dyspnea, activity intolerance Maintaining accurate I & O Daily weights Monitoring Lab values Frequency and consistency of stools Meals include adequate fluid intake Dehydration in the Elderly Increased risks for dehydration: •Decrease in thirst •Lack of fluid replacement •Use of diuretic medications for high BP •Susceptibility to contagious diseases Nursing Care Plan Dehydration • Risk for imbalanced Fluid Volume Related to excessive fluid loss/inability to take in fluids AEB …. Overhydration • Risk for Imbalanced Fluid Volume Related to excessive fluid intake/decreased urination AEB …. Electrolytes – Substance that develops an electrical charge when dissolved in water • Cation - positive charged • Anion - negative charged • Examples of cations: Sodium, Potassium, Magnesium, Calcium • Examples of anions: Chloride, Bicarbonate, Phosphorous Sodium Normal serum values 135-145 mEq/L • Most abundant cation in ECF • Functions – ECF volume – water balance – Acid-base balance – Nerve impulse control – sodium potassium pump – Levels below 115 mEq/L – brain damage, seizures – Sodium is primarily excreted through the kidneys, but other avenues are GI Secretions and sweat. Sodium Deficit - Levels < 135 mEq/L Hyponatremia • Loss through GI tract, skin or kidneys • An increased amount of sodium shift into the cells when there is a potassium deficit • An excessive ADH release (SIADH) causing Water retention and sodium deficit • Inadequate sodium intake, increased water intake • Excessive use of 5% dextrose solution • Levels below 115 mEq/L – brain damage Pathophysiology of decreased sodium imbalances • CNS – excess water moves into the cerebral tissues – increased intracranial pressure • GI – loss causes acid base imbalances • Kidneys – renal dysfunction promotes sodium and water retention resulting in diluted sodium level (fluid overload) • Cellular activity - decrease in Na-K pump Causes of Hyponatremia • Dietary changes – low sodium intake, excessive water intake, “fad diets”/fasting, anorexia nervosa, prolonged use of IV D5W • GI Losses – vomiting, diarrhea, GI Suctioning, Tap water enemas, GI surgery, Bulimia. • Renal Loses – Salt wasting kidney disease, diuretics. • Hormonal Influences - ADH, SIADH. • Decreased adrenocortical hormone: Addison’s disease. • Altered Cellular Function – Hypervolemic state: heart failure, cirrhosis • Burns • Skin Sodium Excess - Levels > 145 mEq/L Hypernatremia • • • • • Excessive secretion of aldosterone or cortisol Excessive sodium intake Decreased water intake GI disorders Decreased renal function Pathophysiology of increased sodium imbalances • Overproduction of adrenal hormones – excessive secretions of aldosterone and cotrisol promote an increase in the sodium level • Cellular activity – increases the sodium pump action, causes cellular irritability. Causes of Hypernatremia • Dietary Changes – Increased sodium intake, decreased water intake, Administration of 3% saline solutions • GI Disorders – severe vomiting, Diarrhea • Decreased Renal Function – reduced glomerular filtration • Environmental Changes – Increased temperature and humidity, water loss • Hormonal Influence – Increased adrenocortical hormone production: oral or IV cortisone or Cushing's syndrome. • Altered Cellular Function – Heart Failure, Renal Diseases • Trauma – Head injury Clinical Manifestations of Sodium Imbalances Hyponatremia • Nausea, vomiting, diarrhea, abdominal cramps • Tachycardia, hypotension • Headaches, apprehension, lethargy, confusion, depression, seizures • Muscle weakness • Dry skin, pale dry mucus membranes • Serum Na <135 mEq/L • Serum osmolality < 275 mOsm/kg • Urine Specific Gravity <1.005 • Urine Sodium >220 mEq/day Hypernatremia • • • • • • • • • • Nausea, vomiting, anorexia Rough, dry tongue Tachycardia, possible hypertension Restlessness, agitation, stupor, elevated body temperature Muscular twitching, tremor, hyperreflexia Flushed, dry skin and dry sticky membranes Serum Na >145 mEq/L Serum osmolality > 295 mOsm/kg Urine Specific Gravity >1.030 Urine Sodium < 40 mEq/day Chloride Normal Levels 95-108 mEq/L • Primary extracellular anion • Creates electrical neutrality when combined with sodium • Body Water Balance • Hydrochloric acid • Buffers carbonic acid • Anion gap - calculated AG = (Na + K) – (Cl + HC03 (metabolic acidosis) Hypochloremia <95 mEq/L • Causes – Loss of gastric fluid – Osmotic diuresis • Manifestations – Reflects alkalosis – Paresthesias, muscle spasms, slow respirations – Dehydration Hyperchloremia > 108 mEq/L • Causes – Hyperparathyroidism, dehydration – Respiratory acidosis • Manifestations – Lethargy, disorientation – Increased rate and depth of respirations Chloride • Primary extracellular anion • Creates electrical neutrality when combined with sodium • Hydrochloric acid • Buffers carbonic acid • Anion gap - calculated AG = (Na + K) – (Cl + HC03 (metabolic acidosis) Potassium Normal serum values 3.5- 5.0 mEq/L • Most abundant cation in ICF • Functions – Transmission and conduction of nerve impulses and the contraction of skeletal, cardiac and smooth muscles (na-K pump) – Assists with regulation intracellular osmolality – Enzyme production for cellular metabolism – Maintains Acid-base Balance – Levels less than 2.5 mEq/L and greater than 7.0 mEq/L can cause cardiac arrest – Potassium is excreted through the kidneys (80-90%) and feces (10-20%) Potassium Deficit - Levels < 3.5 mEq/L Hypokalemia causes • Dietary changes – decrease in dietary intake • Cellular Potassium Loss – Tissue Injury, Muscle contraction • GI losses – vomiting, diarrhea, GI suctioning, intestinal fistula, laxative abuse, bulimia, enemas • Hormonal Influences – Aldosterone (Cushing syndrome), licorice, Stress • Drugs – adrenergic, epinephrine, decongestants, amphotericin B, beta2 –adrenergic agonist, aminoglycosides, large doses of penicillins, potassium wasting diruetics, steroids • Redistribution - Insulin, alkalotic states • Electrolyte loss - Magnesium Potassium Excess - Levels > 5.0 mEq/L Hyperkalemia causes • Dietary – excessive intake, supplements, salt substitutes, herbal juices • IV Potassium replacements – with poor renal function • Decreased renal function – acute and chronic renal failure • Altered Cellular Function – injury, metabolic acidosis, stored blood >1-3 weeks old • Hormonal Deficiency – Addison’s disease • Drugs – K-sparing diuretics, ACE inhibitors, beta blockers • Pseudohyperkalemia – poor blood samples Clinical Manifestations of Potassium Imbalances Hypokalemia • GI – anorexia, N/V, diarrhea, abdominal distention, decreased peristalsis or ileus • Cardiac – dysrhythmias, vertigo (dizziness), cardiac arrest • ECG – Flat or inverted T waves, depressed ST • Renal – polyuria • Neuromuscular – malaise, drowsiness, muscular weakness, confusion, mental depression, diminished deep tendon reflexes, respiratory paralysis • Lab Values - <3.5 mEq/L • Alkalosis Hyperkalema • GI – Nausea, Diarrhea, Abdominal Cramps • Cardiac – tachycardia, then bradycardia and then cardiac arrest • ECG - Peaked T waves, shortened QT interval, prolonged PR followed by a disappearance of the P wave, Prolonged QRS. • Renal - oliguria or anuria • Neuromuscular – weakness, numbness or tingling sensation, muscle cramps • Lab Values - > 5.0 mEq/L • Acidosis Hypokalemia – flattened, inverted T wave with a U wave sometimes present Hyperkalemia – peaked T Wave CLINICAL MANAGEMENT OF POTASSIUM IMBALANCES Hypokalemia • Oral supplements (tablets, capsules, liquid) – Oral potassium is very irritating to the gastric mucosa and should be given diluted and not on an empty stomach • IV Potassium DILUTED in an IV Solution – Never more than 10mEq of KCL per hour – Never given undiluted as a bolus injection – For life threatening hypokalemia (<2.6 mEq/L) 30-40 mEq of KCL can be diluted in 100 – 150 ml of IV Fluid and administered in a central line over an hour. Hyperkalemia • Potassium Restriction • IV Sodium bicarbonate (NaHCO3) – moves K back into the cells – temporary tx • 10% Calcium gluconate – decreases irritability of myocardium, does not promote K loss, use cautiously with patients on digitalis • Insulin and glucose – (10 units of Insulin and 50% dextrose) – Moves K back into the cells • Kayexalate (sodium polystyrene) and sorbitol 70% – Cation exchange • Dialysis Drugs that effect Potassium Balances Hypokalemia • Laxatives and enemas • Corticosteroids • Antibiotics • Potassium-wasting diuretics • Beta2 agonists Hyperkalemia • Oral and intravenous K • Central nervous system agents • Potassium sparing diuretics • ACE inhibitors • Beta blockers • Heparin/Lovenox • NSAIDS Calcium Normal serum values 8.5- 10.5 mg/dL Ionized Calcium 4.0 – 5.0 mg/dL • • • • • • • • Cation found in both ECF and ICF, but greater concentration in ECF Maintains cellular membrane stability 98% in bones and teeth, 2% in the serum Of the 2% in serum - 45% is bound to albumin and 50% is ionized calcium – physiologically active Serum pH greatly affects calcium levels – metabolic acidosis increases ionized calcium levels, alkalosis opposite effect Normal ionized Ca levels are 4.0 to 5.0 mg/dL Serum Ca levels are regulated by Vitamin D, calcitonin (thyroid glad) and parathyroid hormone (PTH) from parathyroid gland There is a direct relationship between Ca and Phosphorous, when Ca is low Phosphorous is high and vise versa. Calcium Regulation When serum Calcium is low: • PARATHYROID GLAND releases PTH. PTH mobilizes calcium from the bone, increases renal reabsorption of calcium and promotes calcium absorption in the intestines in the presence of Vitamin D to increase serum Calcium levels When serum Calcium is High: • THYROID GLAND – releases Calcitonin. Calcitonin increases calcium return to the bone and decreases serum Calcium levels Functions of Calcium • Neuromuscular – normal nerve and muscle activity, causes transmission of nerve impulses and contraction of skeletal muscles. • Cardiac – contraction of the myocardium (heart muscle) • Cellular and Blood – maintenance of normal cellular permeability - decreased calcium increases cellular permeability – Coagulation of blood. Promotes clotting by converting prothrombin into thrombin • Bone and teeth construction – Calcium along with phosphorous forms bones and teeth make them strong and durable Calcium Deficit - Levels < 8.5 mg/dL and Ionized Ca is <4.0 mg/dL Hypocalemia causes • Dietary changes – lack of calcium intake (rare), inadequate Vitamin D, inadequate protein intake, hypoalbuminemia*, chronic diarrhea • Hormone and electrolyte influence – decreased PTH (thyroid surgeries), increased serum phosphorous, decreased magnesium • Calcium Binders or Chelators - citrated blood transfusions • Alkalosis • Increased serum albumin* (low ionized Ca) • Renal Failure – decreases phosphorous excretion and results in excessive Ca Loss • GI Surgery, Pancreatitis and Small Bowel disease Calcium Excess - Levels >10.5 mg/dL and Ionized Ca is >5.0 mg/dL Hypercalemia causes • Primary hyperparathyroidism • Bone malignancy, fractures and immobility • Drug toxicity (lithium carbonate, vitamin a and d, thiazides) • Excessive use of calcium supplements, anti-acids and calcium salts • Renal Impairment and diuretics (thiazides) • Steroid therapy • Decreased serum phosphorous Clinical Manifestations of Calcium Imbalances HYPOCALCEMIA • CNS and Muscular – – – – – – Anxiety, irritability Tetany, muscle twitching (Chvostek’s sign) Numbness and tingling Carpopedal spasm (Trousseau’s sign) Convulsions Abdominal and muscle cramps • Cardiac – Weak contractions – ECG/EKG lengthened ST segment and prolonged QT interval • Blood – reduction in prothrombin (reduced clotting) • Bone – With prolonged deficiency – fractures occur easily HYPERCALCEMIA • CNS and Muscular – Depression/apathy – Weak, flabby muscles • Cardiac – Signs of heart block – Cardiac arrest in systole – Decreased or diminished ST segment and shortened QT interval • Bone – Pathologic fracture – Deep pain over bony areas – Thinning of bones • Renal – Flank pain – Calcium stones in kidney Important Clinical tests for Hypocalemia Chvotsek’s Sign • https://www.youtube.com/ watch?v=ep6IEqnyxJU Trousseau’s Sign • https://www.youtube.com/ watch?v=H13yn9AwtPY&fe ature=relmfu ECG changes with Calcium Imbalances • Normal ST segment and QT Interval • Hypocalcemia – ST segment is lengthened, QT interval is prolonged • Hypercalcemia – ST segment is shortened, QT interval is shortened Clinical Management of Calcium Imbalances Hypocalcemia • Oral supplements and IV calcium diluted in D5W (not normal saline) – IV calcium should be given slowly at 1-3ml/min • Vitamin D supplements Hypercalcemia • Treat underlying cause • IV Normal Saline • Loop Diuretics • Calcitonin SQ • IV phosphates • Others: – Corticosteroids – Antitumor antibiotics MAGNESIUM Normal serum values 1.4- 2.1 mg/dL • Intracellular Cation (2nd most) • Only 1% of body magnesium is in the blood serum the rest is stored in muscle and bone • Of the 1% - 2/3 is ionized (free) and 1/3 is bound to plasma proteins • When calcium absorption goes up magnesium absorption goes down • Alcohol decreases magnesium absorption • Many of the same foods rich in potassium and also rich in magnesium (green vegetable, whole grains, fish, seafood and nuts). • Mg deficiency is usually asymptomatic until <1.0 mg/dL Functions of Magnesium • Neuromuscular activity transmission • Contracts the heart muscle • Cellular – Activates enzymes for carbohydrate and protein metabolism – Responsible for proper transportation of sodium and potassium across cell membranes – Influences utilization of K, Ca, and proteins – Magnesium deficits are FREQUENTLY accompanied by a Potassium and/or Calcium deficit Magnesium deficit < 1.4 mg/dL Hypomagnesemia Causes • Dietary – inadequate intake, mal absorption, GI losses, TPN, malnutrition, starvation, chronic alcoholism, GI fistulas, chronic use of laxatives, chronic diarrhea • Renal Dysfunction – diuresis in diabetic ketoacidosis and in ARF in the diuretic phase • Cardiac dysfunction – post MI, prolonged diuretic therapy with CHF • Electrolyte and Acid/Base Influences – hypocalcemia, hypokacemia, metabolic alkalosis • Drug influence – potassium wasting diuretics, aminoglycosides, cortisone, amphotericin B, digitalis Magnesium excess > 2.1 mg/dL Hypermagnesemia Causes • Dietary – prolonged use/excessive use of magnesium-containing anta-acids (Maalox), laxatives (MOM) and IV magnesium sulfate • Renal Dysfunction – Renal insufficiency and failure • Severe Dehydration – Diabetic ketoacidosis Clinical Manifestations of Magnesium Imbalances Hypomagnesemia • Neuromuscular – hyperirritability, tetany-like symptoms, tremors, twitching of the face, spasticity, increased tendon reflexes • Cardiac – Hypertension, cardiac dysrhythmias PVC’s, VT (Torsades), VF, and flat or inverted T wave, ST depression (like low K levels) Hypermagnesemia • Neuromuscular – CNS depression, lethargy, drowsiness, weakness, paralysis, loss of deep tendon reflexes • Hypotension, Complete heart block (3rd degree), bradycardia, Widened QRS complex, prolonged QT interval • Others: Flushing, respiratory depression Hypomagnesemia Clinical Management of Magnesium Imbalances Hypomagnesemia • Oral or IV replacements • Diet high in magnesium green vegetables, whole grains, legumes, nuts Hypermagnesemia • Correct the underlying cause • IV saline or calcium • Dialysis Phosphorous Normal levels 2.5 – 4.5 mg/dL • Major Intracellular anion • Needed for metabolism, nerve and muscle function • Part of energy units • Component of phospholipids (cellular and organelle membranes) • Regulated by calcitonin, parathyroid hormone AND Vitamin D • Levels vary with acid-base balance • Glucose, insulin or sugar-containing foods temporarily shift phosphorous into the cells Functions of Phosphorous • Neuromuscular – normal nerve and muscle activity • Bones and teeth – bone and teeth formation, strength and durability • Cellular – forms high energy compounds (ATP, ADP, AMP), is the backbone of nucleic acids and stores metabolic energy – Formation of red blood cell enzyme (2,3diphosphoglycerate) that delivers oxygen to tissues – Utilization of B vitamins – Metabolism of fats, carbohydrates and proteins – Maintenance of ACID BASE balance in body fluids Phosphorous deficit < 2.5 mg/dL Hypophosphatemia Causes • Dietary - Vitamin D deficiency, chronic alcoholism TPN • GI – malabsorption, vomiting, diarrhea • Hormonal Influence – Hyperparathyroidism (increased PTH) • Drugs – aluminum containing antacids (binders), diuretics • Cellular – Diabetic Ketoacidosis • Acid-Base disorders – Respiratory alkalosis, metabolic alkalosis Phosphorous excess > 4.5 mg/dL Hyperphosphatemia Causes • Dietary – excessive administration of phosphorous containing substances • Hormonal – lack of PTH • Renal Insufficiencies – Renal Failure (ARF, CRF) • Drug – frequent use of phosphate laxatives • Cellular destruction – chemo, radiation, rhabdomyolsis (breakdown of striated muscle) • Acid-Base disorders – Metabolic and Respiratory Acidosis Clinical Manifestations of Phosphorous Imbalances Hypophosphatemia • • • • Hyperphosphatemia Neuromuscular – muscle • Neuromuscular – Tetany (with weakness, tremors, decreased calcium), paresthesia, bone pain, hyporeflexia, seizures, hyperreflexia, muscular delirium, hallucinations, weakness (more common with ascending motor paralysis hypophosphatemia), flaccid Hematologic – tissue hypoxia, paralysis possible bleeding, possible infection • Cardiopulmonary – Cardiopulmonary – weak tachycardia pulse, hyperventilation, respiratory weakness • GI- nausea, diarrhea, GI – Anorexia, dysphagia abdominal cramps Clinical Management of Phosphorous Imbalances Hypophosphatemia • Oral phosphorous replacements • Treat underlying causes • Diet high in phosphorous • Avoid phosphorous binding antacids • IV phosphorous (only when levels are below 1 mg/dl) dose 12-15 mmol/L diluted in IV fluid Hyperphosphatemia • Phosphorous binding antacids • Calcium based antacids are preferred in renal failure to avoid hypermagnesemia • If hyperphosphatemia is accompanied by hypocalcemia correcting calcium level will reduce phosphorous levels • Administer Insulin and glucose ACID BASE IMBALANCES NURS 2140 Winter 2012 Teresa Champion, RN, MSN ACID BASE BALANCE • Hydrogen ions - Low concentrations but highly reactive – Concentration affects physiological functions, for example: • Alters protein and enzyme functioning • Can cause cardiac, renal, respiratory abnormalities • Alters blood clotting, metabolization of meds Acid and Bases • Acids – compounds that form hydrogen ion in a solution – Proton donors – Strong acids give up their hydrogen ion easily – Weak acids hold on to their hydrogen ion more tightly • Bases – compounds that combine with hydrogen ion in a solution – Proton acceptors – Neutralizes • 20:1 ratio (20 parts bicarbonate to one part carbonic acid) The Basics explained: • pH is a measurement of the acidity or alkalinity of the blood. • It is inversely proportional to the number of hydrogen ions (H+) in the blood. – The more H+ present, the lower the pH will be. – The fewer H+ present, the higher the pH will be. Plasma pH • Inversely related to hydrogen ion concentration – ↑ hydrogen ion concentration, ↓ pH – ↓ hydrogen ion concentration, ↑ pH Body Acids • Respiratory Acid - CO2 – eliminated by lungs (daily ~288L/day) • Metabolic acids: (either excreted by kidney or metabolized in liver and Production: 0.1 mol (100 mEq)/day. Eliminated more slowly than respiratory acid – – – – – Lactic acids Pyruvic acid Ketoacids (DKA) Acetoatic acids Beta-hydrobutyric acids Normal Blood pH • The normal blood pH range is 7.35 to 7.45. – Slightly alkalotic – Must maintain this range for normal body functions • < 7.35 Blood pH is acidotic • > 7.45 blood pH is alkalotic • This is the FIRST step in interpretation of Arterial Blood Gases and plasma pH. BUFFER SYSTEMS • Extracellular Buffers – carbonic acid (lungs) and bicarbonate (kidneys) • Intracellular Buffers – Phosphate Buffer System • Dihydrogen phosphate (H2PO4) – hydrogen donor • Hydrogen phosphate (HPO4) – hydrogen acceptor • Protein Buffers – Plasma Proteins – Hemoglobin – oxyhemoglobin and deoxygenated Hgb • Bones – Carbonate and phosphate salts in bone provide a long term supply of buffer. Role of the lungs • Regulate plasma pH minute to minute by regulating the level of Carbon Dioxide (CO2) • Carbon Dioxide is measured as a partial pressure of carbon dioxide in arterial blood – PaCO2 35-45mmHg • Lungs alter rate and depth of ventilations in order to retain or excrete CO2 Minute Volume – Tidal Volume • Ventilation is measured by how much air the lungs move in one minute (minute ventilation) • Minute Ventilation is the product of respiratory rate and depth and is referred to as the TIDAL VOLUME (Vt) • NORMAL tidal volume at rest is about 500ml. • Aveolar volume = tidal volume – anatomical dead space • Aveolar minute ventilation = respiratory rate x alveolar volume Terms for Respiration • Hypercarbic Drive – increased PaCO2 levels raise the level of H+ ions, lowering pH. Central Chemorectptors (in CNS) stimulate the phrenic nerve, increasing respiration • Hypoxic Drive – peripheral chemoreceptors in carotid arteries and arch of aorta are stimulated by low PaO2 level (<60mmHg) increasing respiratory rate. • Hypocapnia – Low PaCO2 (<35) • Hypercapnia – High PaCO2 (>45) The role of the Kidneys • Two main functions to maintain acid/base – Secrete hydrogen ions – Restore or reclaim bicarbonate • Secretion – active process of moving substances from blood into the tubular fluid against a concentrated gradient, for example hydrogen ions. • In high metabolic acidosis, the kidneys can excrete ammonia as a urinary buffer. • In alkalosis - the kidneys retain hydrogen ion and excrete bicarbonate to correct the pH. • In acidosis - the kidneys excrete hydrogen ion and conserve bicarbonate to correct the pH. Assessment of ACID BASE • Arterial Blood Gases (ABG) most often and the most accurate to assess acid base balances. • Serum Electrolytes can help fine tune acid base analysis • NORMAL ABG VALUES: • pH = 7.35 to 7.45 • PaCO2 = 35 – 45 mEq/L (40 mEq/L – middle range) • HCO3 = 22 – 28 mEq/L Steps to Interpret ABG’s 1. Assess the pH 2. Assess the respiratory component – PaCO2 3. Assess the metabolic component – HCO3, base excess 4. Evaluate compensation Respiratory Acidosis • High PaCo2 (>45 mEq/L) with resultant drop in in pH (<7.35 - acid) • Respiratory system fails to eliminate the appropriate amount of carbon dioxide to maintain the normal acid-base balance • Caused by pneumonia, drug overdose, head injury, chest wall injury, obesity, asphyxiation, drowning, or acute respiratory failure • Medical treatment – Improve ventilation, which restores partial pressure of carbon dioxide in arterial blood (Paco2) to normal Respiratory Acidosis • Nursing care – Assess Paco2 levels in the arterial blood, and pH. – Observe for signs of respiratory distress: restlessness, anxiety, confusion, tachycardia • Intervention – Encourage fluid intake – Position patients with head elevated 30 degrees Respiratory Alkalosis • Low PaCO2 (< 35 mEq/L) with a resultant rise in pH (>7.45 – alkalotic) – Most common cause of respiratory alkalosis is hyperventilation – usually caused by pain, anxiety • Medical treatment • Major goal of therapy: treat underlying cause of condition; sedation may be ordered for the anxious patient • Nursing care – Intervention • In addition to giving sedatives as ordered, reassure the patient to relieve anxiety • Encourage patient to breathe slowly, which will retain carbon dioxide in the body Metabolic Acidosis • Body retains too many hydrogen ions or loses too many bicarbonate ions; with too much acid and too little base, blood pH falls (pH <7.35) • Causes are starvation, dehydration, diarrhea, shock, renal failure, and diabetic ketoacidosis • Signs and symptoms: changing levels of consciousness, headache, vomiting and diarrhea, anorexia, muscle weakness, cardiac dysrhythmias • Medical treatment: treat the underlying disorder Metabolic Acidosis cont’d • Nursing care – Assessment of the patient in metabolic acidosis should focus on vital signs, mental status, and neurologic status – Emergency measures to restore acid-base balance. Administer drugs and intravenous fluids as prescribed. Reassure and orient confused patients Metabolic Alkalosis • Increase in bicarbonate levels or a loss of hydrogen ions and increases pH (>7.45) • Causes: – Loss of hydrogen ions may be from prolonged nasogastric suctioning, excessive vomiting, diuretics, and electrolyte disturbances • Signs and symptoms: – headache; irritability; lethargy; changes in level of consciousness; confusion; changes in heart rate; slow, shallow respirations with periods of apnea; nausea and vomiting; hyperactive reflexes; and numbness of the extremities • Medical treatment – Depends on the underlying cause and severity of the condition Metabolic Alkalosis • Nursing care – Assessment • Take vital signs and daily weight; monitor heart rate, respirations, and fluid gains and losses • Keep accurate intake and output records, including the amount of fluid removed by suction • Assess motor function and sensation in the extremities; monitor laboratory values, especially pH and serum bicarbonate levels • Intervention – To prevent metabolic alkalosis, use isotonic saline solutions rather than water for irrigating nasogastric tubes because the use of water for irrigation can result in a loss of electrolytes