Body Fluid and Electrolytes
advertisement

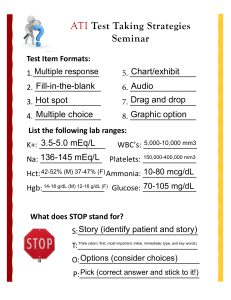
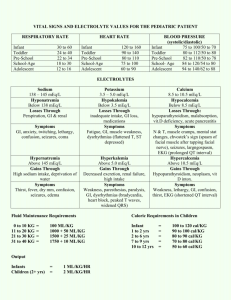
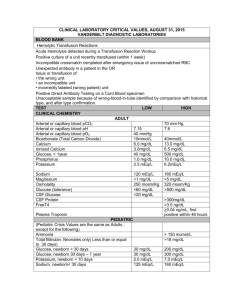
CRC 435 Cardiorespiratory Care University of South Alabama Describe the distribution of body water Describe how body water is regulated Describe how body water is transported among body compartments State and explain Starling’s Law of the Capillaries List the major electrolytes, their normal concentrations, the causes of abnormalities, and symptoms of abnormalities 45%-80% of body weight, depending on gender, age, and weight Water is distributed as intra and extra cellular Extracellular water is divided into intravascular, interstitial, and transcellular fluids Important transcellular fluids are pleural fluid and ascites The predominant extracellular electrolytes are sodium, chloride and bicarbonate 11-15% <1% 45% of body weight 15-20% The predominant intracellular electrolytes are potassium, Mg, phosphate, and sulfate Plasma has more protein and is responsible for osmotic pressure Kidneys maintain the volume and composition of body fluids by filtration and reabsorption of sodium by secretion of antidiuretic hormone (vasopressin) Water loss is insensible (skin & lung) or sensible (urine, intestinal, sweat) burn patients have huge insensible losses humidifiers are necessary to make up for these losses when an artificial airway is present Qf = K1(Pch - Pih) - K2(Pco - Pio) Hydrostatic pressure * * Osmotic pressure when hydrostatic pressure (heart failure) is very high or when osmotic pressure is very low (protein starvation, low albumen), or if the lymphatics are incompetent, edema occurs * Ernest Starling (1866-1927) Sodium regulation goes along with body water found in extracellular fluid (50%), bone (40%) and cells (10%) normal is 136-145 mEq/L causes and symptoms of abnormal values Chloride most prominent anion; 67% is extracellular normal is 98-106 mEq/L Bicarbonate primary means for CO2 transport from tissues to lungs maintains pH normal is 22-26 mEq/L Potassium major intracellular cation, 98% is in cells exchanges with sodium and hydrogen ions by active transport normal is 3.5 -5 mEq/L may be lost due to surgery, trauma, or renal disease, requiring replacement, usually as KCl Calcium mediator of neuromuscular function and cell enzyme processes most is in bone normal is 8.7 - 10.4 mg/dL or 4.5 - 5.25 mEq/L maintained by parathyroid hormone, vitamin D, and calcitonin Magnesium intracellular cation roles in cellular functions: energy transfer, metabolism of substrates; maintains cell membrane function normal is 1.7-2.1 mg/dL or 1.7 - 1.4 mEq/L Phosphorus 80-90% in bone or teeth role in metabolism of cellular energy; source of phosphate for ATP 1.2 - 2.3 mEq/L kept balanced by GI absorption and urinary secretion regulated by parathyroid hormone