ionic bond

Mr. Shields Regents Chemistry U09 L01
1
Elements found in Abundance in the Earth’s Crust
Only Five Elements account for 93% of the mass of
The earth’s crust
What do you think they might be?
5 elements make up
93% of the earths crust
And only 8 make up
99% of the entire mass
But consider the diversity of compounds in our world! How
Can there be so many different combinations?
2
Types of Bonds
Compound diversity found in the world is a consequence of the many possible ways elements can combine with one another.
Elements combine by forming what is called the
CHEMICAL BOND
So … What is a Chemical Bond?
“ A Chemical Bond is a FORCE OF ATTRACTION holding two or more atoms together”
3
Types of Bonds
There are several different types of Chemical Bonds:
Ionic
Metallic
Network
Covalent
Polar Covalent
All these bond types involve ONLY the electrons in the atoms valence shell, i.e. the valence electrons
4
Ionic Bonds
In this unit we’ll discuss Ionic compounds first.
Everyone is familiar with STATIC ELECTRICITY
- Such as when you get a shock touching a doorknob after walking across a rug in the winter
- In this case you’re neg. and the doorknob is +
- Electrons are transferred in the process
- So… From what to what?
5
Ionic Bonds
Static electricity is also the basis for ionic bond formation
In Ionic bonds there are metal atoms & non-metal atoms
- We know metals want to lose electrons and
Non-metals want to gain electrons. WHY?
Remember effective nuclear charge?
e-
- During the formation of IONIC BONDS metals
TRANSFER valence electrons to the non-metal
6
Ionic bonds are formed by the
Attraction between positive and negative ions
7
Electron Transfer between Sodium and Chlorine
2Na + Cl
2
2NaCl
How many electrons are transferred?
And from where to where?
8
We know the valence electrons are involved in electron transfer to form the ionic bond
BOTH NOW HAVE AN OCTET
But WHICH electrons (i.e. what orbitals) are involved?
Formation of
The Octets
A quantum mechanical representation of the electron transfer
9
Remember that the number of valence electrons an atom has determines its OXIDATION NUMBER
What are
The oxidation
Nos. of
These Atoms?
10
Group 1 & 17
The oxidation number determines how many atoms
Are needed to form the ionic compound
Ox. No: +1 -1 +1 -1
Chemical Formula: M
1
X
1
(i.e. MX) -WHY?
11
Group 2 & 17
Ox. No: -1 +2 -1
Chemical Formula: M
1
X
2
(i.e. MX
2
) - WHY?
12
Group 13 & 16
Ox. No: -2 +3 -2 +3 -2
Chemical Formula: M
2
X
3
– WHY?
13
Ionic Bonds
So… The driving force for ionic bond formation is the
Attainment of the octet
Remember:
Metals with Low IE lose electrons more readily than non-metals with higher IE
Non-metals with High Electronegativities attract electrons more readily than metals with Lower Electronegativities
14
And… for Metals lower Ionization energy means H igh Reactivity .
For Non-metals lower Electronegativity means Lower Reactivity .
Therefore Francium and Fluorine are the most Reactive elements.
Dec.
I.E.
Na
Increasing
Reactivity
Dec.
EN
F
Decreasing
Reactivity
Fr I
IE and EN decreases down a group and…
IE and EN both increase L to R across a period
15
Electronegativity Difference
The ability to fully transfer or accept electrons
Defines ionic bonds we can determine which bonds are
More ionic than others by calculating the difference in
EN Between the Atoms
For Example
When the difference in
Electronegativity
Between 2 elements
Is greater than 1.7
the
Elements will form an
Ionic bond
Non-ionic ionic ionic
Fr + F -
EN Diff = 3.3
Most ionic compound
16
Least Ionic -
Most covalent
2.1
Most Ionic –
Least covalent
3.2
1.7
Diatomic molecules Like H
2
, O
2
, Cl
2 etc ∆EN =0
17
We saw that when Na and Cl
2 amount of heat released react there is a large
We’ve burned Mg in air (O this reaction there is also a lot of
Heat & light released
2
) and saw that in
All IONIC REACTIONS are
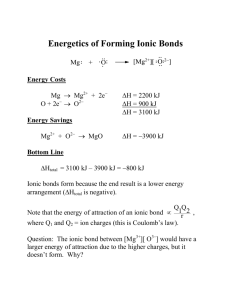
Characterized by the release of heat i.e. they are EXOTHERMIC
Reaction of sodium metal and chlorine gas
18
Bond Formation
When we release heat we are releasing energy
Therefore ionic reaction products have less energy
Than the starting reactants
- Decreasing energy means greater stability
Bonds have
An ideal bond
Length.
Na + Cl
Potential Energy is
Stored in the
Chemical Bond
Push atoms
Closer together
& PE inc.
NaCl
19