System of Equations
advertisement
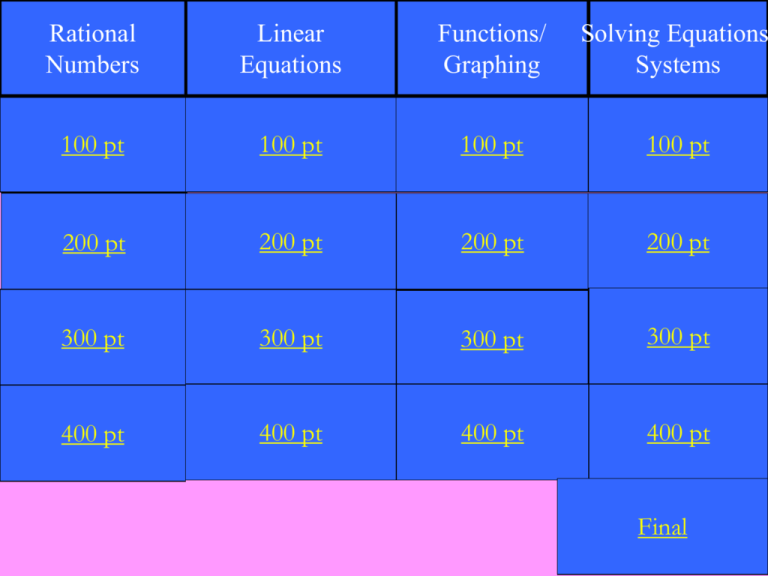
Rational Numbers Linear Equations Functions/ Graphing Solving Equations Systems 100 pt 100 pt 100 pt 100 pt 200 pt 200 pt 200 pt 200 pt 300 pt 300 pt 300 pt 300 pt 400 pt 400 pt 400 pt 400 pt Final Which number is irrational? A. 213 2 B. 3.12 C. 64 D. 9.31307... D. 9.31307… Which is the best approximation of A. 7 B. 7.41 C. 7.42 D. 8 55 ? C. 7.42 33 is between two integers. What are the squares of those integers? 25 and 36 Suppose 17.12 is graphed on the number line. What square root would be graphed at that same point on the number line (round to the nearest whole number)? 293 Which equation is a linear equation? 2 x a. y = + 3 b. xy = 5 c. y = 2/3x d. x = -9 d. x = -9 At what point will the line 2x - 3y = 7 cross the x-axis? (3½ , 0) Write the linear equation in standard form: 2 1 x y 3 2 4x - 6y = -3 Write an equation in slope intercept form for a line passing through (2, 0) and (-1, 6). y = -2x+4 Which ordered pair represents a point that would lie on the graph of y = 4x -10? A. (4, -10) B. (4, 6) C. (-4, 6) D. (-4, 10) B. (4, 6) 2 -2x If g(x) = + 4x + 9, find g(-3). -21 Write an equation of the line in slope intercept form. y = - 2x + 4 Graph on your whiteboard 3x – 4y = 8 A system of equations produces two lines that intersect. The solution of the this system can be described as…. One point Solve the system using substitution 1 x = 2 y+3 2x – y = 6 Infinitely many solutions Solve the equation 4 1 2 x 4 1 x 3 x 3 3 x=6 Solve the system using elimination 3x + 4y = -25 2x - 3y = 6 (-3, -4) FINAL JEOPARDY CHEMISTRY Michael needs 10 milliliters of 34% HCl (hydrochloric acid) solution for a chemistry experiment. There is a bottle of 10% HCl solution and a bottle of 40% HCl solution in the lab. How much of each solution should he use to obtain the required amount of 34% HCl solution? 2 mL of the 10% HCl solution 8 mL of the 40% HCl solution CHEMISTRY Michael needs 10 milliliters of 34% HCl (hydrochloric acid) solution for a chemistry experiment. There is a bottle of 10% HCl solution and a bottle of 40% HCl solution in the lab. How much of each solution should he use to obtain the required amount of 34% HCl solution? (Think back to chapter 2… this time we will use 2 equations instead) Given information 10 mL 34% HCL x mL 10% HCL y mL 40% HCL Solve y = 10 – x 0.10x + 0.40(10 – x) = 3.4 0.1x + 4 – 0.4x = 3.4 -0.3x = -6 x=2 System of Equations x + y = 10 0.10x + 0.40y = 0.34(10) y = 10 – x y = 10 – 2 y=8 Answer: 2 mL of 10% HCl solution, 8 mL of 40% HCl solution






