Chemistry: Ch
advertisement
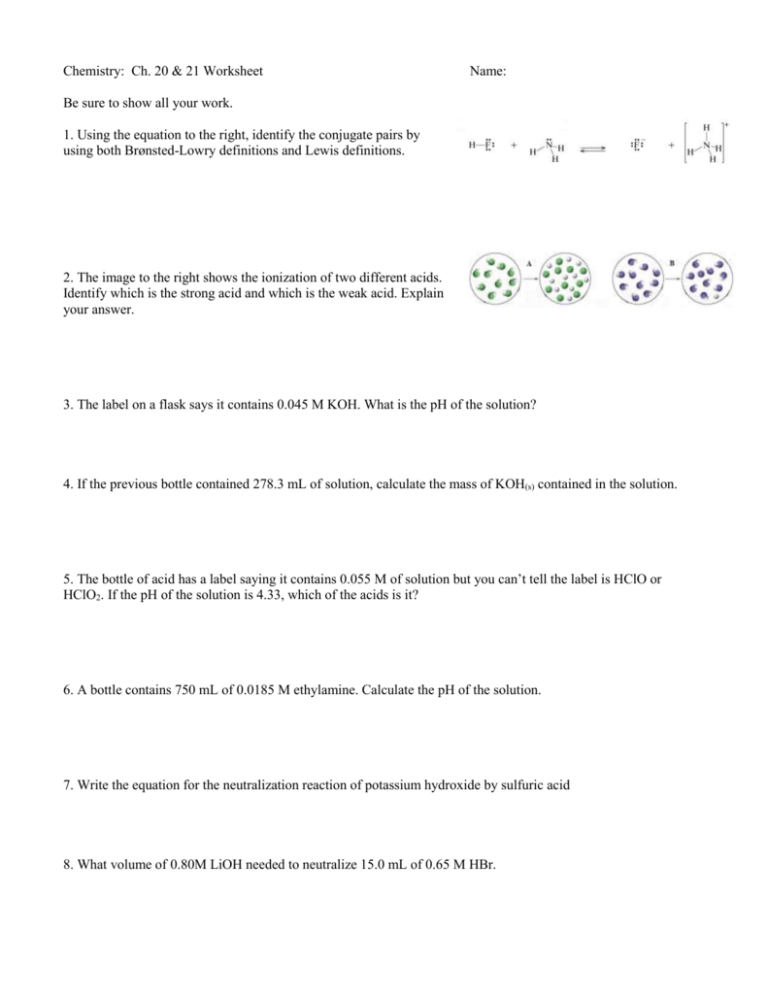
Chemistry: Ch. 20 & 21 Worksheet Name: Be sure to show all your work. 1. Using the equation to the right, identify the conjugate pairs by using both Brønsted-Lowry definitions and Lewis definitions. 2. The image to the right shows the ionization of two different acids. Identify which is the strong acid and which is the weak acid. Explain your answer. 3. The label on a flask says it contains 0.045 M KOH. What is the pH of the solution? 4. If the previous bottle contained 278.3 mL of solution, calculate the mass of KOH(s) contained in the solution. 5. The bottle of acid has a label saying it contains 0.055 M of solution but you can’t tell the label is HClO or HClO2. If the pH of the solution is 4.33, which of the acids is it? 6. A bottle contains 750 mL of 0.0185 M ethylamine. Calculate the pH of the solution. 7. Write the equation for the neutralization reaction of potassium hydroxide by sulfuric acid 8. What volume of 0.80M LiOH needed to neutralize 15.0 mL of 0.65 M HBr. 9. It requires 24.6 ml of Ca(OH)2 solution to neutralize 14.2 mL of 0.0140M H HCl. What is the concentration of the calcium hydroxide solution? 10. A 55.0 g sample of Al(OH)3 is reacted with 0.200 M HCl. How many mL of the acid are needed to neutralize the Al(OH)3? 11. Calculate the final pH of a solution containing 22.5 mL of 0.010 M HCl and 35.0 mL of 0.005 M NaOH 12. Calculate the pH of a 5.00 x 103 mL solution containing 68.9 g of KOH and 125.0 g of HI. 13. The image to the right contains a laboratory setup for finding the concentration of the “unknown acid”. Explain all the things you will have to do in order to find the concentration.











