MIT 8.044 Statistical Physics I: Thermodynamics Lecture Notes
advertisement
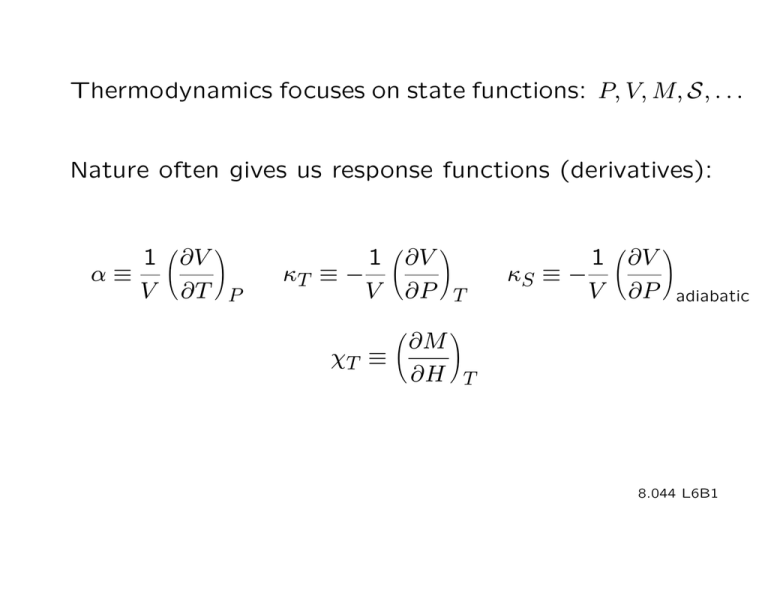
Thermodynamics focuses on state functions: P, V, M, S, . . . Nature often gives us response functions (derivatives): 1 ∂V α≡ V ∂T P � � 1 ∂V κT ≡ − V ∂P T � � 1 ∂V κS ≡ − V ∂P � � adiabatic ∂M χT ≡ ∂H T � � 8.044 L6B1 Example Non-ideal gas Given • Gas → ideal gas for large T & V • ∂P Nk = ∂T V V − Nb • ∂P N kT 2aN 2 =− + 2 ∂V T (V − N b) V 3 Find P 8.044 L6B2 � dP = P = ∂P ∂P dV + dT ∂V T ∂T V � � � ∂P � ∂T V � � dT + f (V ) = � � Nk V − Nb � dT + f (V ) N kT = + f (V ) (V − N b) 8.044 L6B3 2 ∂P N kT N kT 2aN I(V ) = − = − + f + j V ) 2 2 3 ∂V T (V − N b) (V − N b) j V V ) f (V ) = � 2aN 2 aN 2 dV = − 2 + c 3 V V N kT aN 2 P = − 2 + c (V − N b) V but c = 0 since P → N kT /V as V → ∞ 8.044 L6B4 Internal Energy U Observational fact initial ∆W final isolated (adiabatic) Final state is independent of how ΔW is applied. Final state is independent of which adiabatic path is followed. 8.044 L6B5 ⇒ a state function U such that ΔU = ΔWadiabatic U = U (independent variables) = U (T, V ) or U (T, P ) or U (P, V ) for a simple fluid 8.044 L6B6 Heat If the path is not adiabatic, dU = d /W d /Q ≡ dU − d /W d /Q is the heat added to the system. It has all the properties expected of heat. 8.044 L6B7 First Law of Thermodynamics dU = d /Q + d /W • U is a state function • Heat is a flow of energy • Energy is conserved 8.044 L6B8 Ordering of temperatures dQ T1 T2 When d /W = 0, heat flows from high T to low T. 8.044 L6B9 Example Hydrostatic System: gas, liquid or simple solid Variables (with N fixed): P, V, T, U . Only 2 are independent. ⎛ ⎞ d /Q ⎠ CV ≡ dT V ⎝ ⎛ ⎞ d /Q ⎠ CP ≡ dT P ⎝ Examine these heat capacities. 8.044 L6B10 dU = d /Q + d /W = d /Q − P dV d /Q = dU + P dV d We want . We have dV . dT ∂U ∂U dU = dT + dV ∂T V ∂V T � � � � 8.044 L6B11 ∂U d /Q = dT + ∂T V �� ∂U + P dV ∂V T d /Q ∂U ⇒ = + dT ∂T V �� ∂U dV +P ∂V T dT � � � ⎛ � ⎞ � � � � d /Q ⎠ ∂U CV ≡ = dT V ∂T V � � ⎝ 8.044 L6B12 ⎛ ⎞ d /Q ⎠ ∂U ⎝ CP ≡ = + dT P " ∂T T Vv � � �� ∂U +P ∂V T CV �� CP − CV = � ∂V " ∂T T Pv �� � αV ∂U + P αV ∂V T � � The 2nd law will allow us to simplify this further. ∂U Note that CP = . ∂T P � � 8.044 L6B13 Paths Experimental conditions, not just math fills container ∆V=0 floating piston ∆P=0 bath insulation ~~~~~~~ ∆T=0 ∆Q=0 8.044 L6B14 8.044L12B1 ∆Q = 0 could come from time considerations Example Sound Wave ρ(x) v x too fast for heat to flow out of compressed regions v= 1 ρκS 8.044 L6B15 8.044L12B2 Example Hydrostatic system: an ideal gas, PV=NkT New information ! ∂U =0, ∂V T 3 possible sources Experiment free expansion bath initially at Ti observe Tf = Ti 8.044 L6B16 8.044L12B3 No work done so ΔW = 0 Tf = Ti ⇒ ΔQ = 0 together ⇒ , ΔUU== 0Q here → (∂U/∂VU=)T = 0Q quasi-static changes , • Physics: no interactions, single particle energies only ⇒ (∂U/∂V )T = 0 • Thermo: 2nd law + (P V = N kT ) ⇒ (∂U/∂V )T = 0 8.044 L6B17 Consequences ∂U ∂U dU = dT + dV , ∂T vt Vv , ∂V vt Tv � � CV U = � � 0 � T CV (T ') dT ' + constant , vt v 0 set=0 In a monatomic gas one observes CV = 3 2 N k. Then the above result gives U = CV T = 3 2 N kT . 8.044 L6B18 ∂U CP − CV = ( +P ) , ∂V Tp � � 0 = Nk ∂V , ∂T Pp � � ∂ (N kT /P ) =N k/P P ∂T for any ideal gas Applying this to the monatomic gas one finds 3 5 CP = N k + N k = N k 2 2 5 γ ≡ CP /CV = 3 8.044 L6B19 Adiabatic Changes d /Q = 0 Find the equation for the path. Consider a hydrostatic example. ∂U ∂U d /Q = dT + + P dV = 0 T � ∂T �� V� � ∂V �� � � � �� CV � � � (CP −CV )/αV ⎛ ⎞ (γ − 1) ∂T CP − CV ) ⎠ 1 ⎝ =− =− ∂V ΔQ=0 CV αV αV � This constraint defines the path. 8.044 L6B20 Apply this relation to an ideal gas. 1 ∂ N kT 1 Nk 1V 1 1 ∂V α≡ = = = = V ∂T P V ∂T P V P VT T P � � � � � � Path dT T = −(γ − 1) dV V ⎛ ⎞ dT dV T⎠ V ⎝ = −(γ − 1) → ln = −(γ − 1) ln T V T0 V0 ⎛ ⎝ ⎞ T⎠ = T0 ⎛ ⎝ ⎞−(γ−1) V ⎠ V0 8.044 L6B21 Adiabatic Isothermal Adiabatic 1=c γ γ− T V 1 =c TV Isothermal P "" P V = c ′′ PV = c adiabat −1 γ γ ′ c" PP V V== c γ = 5/3 (monatomic) PP∝∝ V V1 isotherm 5/3 P γ∝= V 5/3 (monatomic) dP = dV 5P 3V P ∝ V −5/3 dP 5P =− dV 3V dP P =− dV P V dP dV = V V 8.044L12B9 8.044 L6B22 Expansion of an ideal gas insulation F rupture diaphragm adiabatic ∆Q = 0 not quasistatic ∆W = 0 ∆U = 0 T slowly move piston adiabatic ∆Q = 0 quasistatic ∆W is negative ∆U = is negative T adiabat constant U V V 8.044 L6B23 8.044L12B10 Starting with a few known facts, /W , and state function math, 1st law, d one can find relations between some thermodynamic quantities, a general expression for dU , and the adiabatic constraint. Adding models for the equation of state and the heat capacity allows one to find the internal energy U and the adiabatic path. 8.044 L6B24 MIT OpenCourseWare http://ocw.mit.edu 8.044 Statistical Physics I Spring 2013 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.