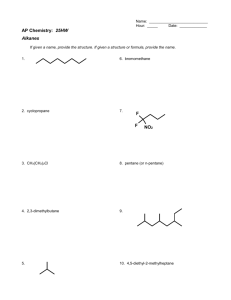
Which is Aromatic?
advertisement

Aromatic Chemistry, Amines & Natural Products Dr. Fawaz Aldabbagh NUI, Galway Fawaz.Aldabbagh@nuigalway.ie Recommended Text: Organic Chemistry by Paula Y. Bruice 4th or 5th Edition Benzene First isolated by Michael Faraday in 1825 H H H C C C C C C H H H General Formula: C6H6 Aromatic originally referred to the smell, as opposed to aliphatic Test for Aromatic: High Carbon content – burn with a smoky flame Structure of Benzene 1. Each Carbon atom is sp2-hybridised : Making three σ-bonds with two adjacent carbons and one hydrogen atom: bond angles = 120º This is the σ-Framework 2. The p-orbital on each carbon atom overlaps above and below the σ-framework with the p-orbital on the adjacent carbon atom H H H C C C C H C C H H 1856: Friedrich Kekulé Resonance Structures Resonance arrow indicates structures are electronically equivalent The problem is that C-C single and double bonds would be different lengths and molecule would be a distorted hexagon 1930s: X-ray crystal structures showed benzene is planar and six carboncarbon bonds have the same length (C-C 1.39 A), which is shorter than C-C single bond at 1.54 A. But longer than a C=C. Thus, benzene does not have alternating bonds! • • • Kekulé resonance structures Neither canonical form is correct structure Resonant Hybrid is correct structure H H H H H C C C C C C H H H H H C C C C C C H H H H H H C C C C C C H H Another Problem with the Kekulé Model: Extra Stability than 1,3,5-Cyclohexatriene Structure Would Suggest! + H2 -29 kcalmol-1 + 3 H2 -87 kcalmol-1 (Calculated) Experimental ∆Hº = 50 kcalmol-1 Benzene is more stable than expected, and cannot be cyclohexatriene. The six π-electrons must be delocalised and not localised 37 kcalmol-1 of resonance stabilization Delocalisation of the six π-electrons A resonance hybrid is more stable than the predicted stability of its resonance contributor, and the more resonance contributors there are, the greater the stability p-orbitals must be aligned! When Is A Molecule Aromatic? • For a molecule to be aromatic it must: Be cyclic Have a p-orbital on every atom in ring Be planar Posses 4n+2 p electrons (n = number of rings) benzene naphthalene Erich Hückel phenanthrene Aromatic Hydrocarbons Aromatic Molecules? • Are the following structures aromatic? Cyclic? P-Orbital? Planar? 4n+2 p electrons? Cycloheptarienyl Cycloheptarienyl cation H anion H + C C [14]-Annulene Cyclobutadiene No Yes No Yes Which is Aromatic? Draw Resonance Structures if so! + _ Homework: Try Problem 4 : page 645 of Bruice (5th Ed) or Problem 2 : page 597 of Bruice (5th Ed) Aromatic Hydrocarbons: Nomenclature • Substituent plus benzene However, there are exceptions CH3 Ph Methylbenzene (Toluene) OH CH3 Cl Ph Chlorobenzene NH2 NO2 Cl Ph NO2 Nitrobenzene O Benzoic acid OH Phenol Ph OH Aniline Ph NH2 OMe Ph CO2H Ph OMe Methoxybenzene (Anisole) Nomenclature • Two identical substituents: di- (3: tri- etc.) • Numbering of C atoms in ring 6 6 CH3 6 CH3 CH3 5 1 5 1 5 1 4 2 4 2 4 2 3 CH3 1,2-Dimethylbenzene o-Dimethylbenzene o-Xylene (o: ortho) 3 CH3 1,3-Dimethylbenzene m-Dimethylbenzene m-Xylene (m: meta) H3C 3 1,4-Dimethylbenzene p-Dimethylbenzene p-Xylene (p: para) Electrophilic Aromatic Substitution H E H H E H X H + H H H H H X H H Electrophilic attack – Slow Rate Determining Step E H E H E H E sp3 Transition State or Wheland Intermediate E H Delocalised Cyclohexadienyl cation Fast Step is the loss of a proton + E ---rapid re-aromatization - H+ E H E.g. Nitration of benzene HNO3(c), H2SO4(c) NO2 + H+ Sir Christopher Ingold's ideas (1930s), terminology and nomenclature for reaction mechanisms (e.g. electrophilic, nucleophilic, inductive, mesomeric, SN1, SN2 etc) were generally accepted and employed everywhere. Derive the Lewis Structure of NO2+, which is the electrophile (E+) for nitration Generating NO2+ Sulfuric acid is a stronger acid than nitric acid O _ H O S O H O _ O S O + 2 H+ O O H HO NO2 H+ H O+ NO2 NO2+ + H2O The Nitration of Benzene _ O O + N electrophilic attack O electrophile O2 N + O +N + slow H - H+ fast O + N _ O O +N = O NO2 = + Label the parts of the Electrophilic Aromatic Substitution Profile Some Reactions of Benzene You should be able to write mechanisms for all the transformations below. All reactions will be described in your lectures Br2, FeBr3 (cat) Cl2, FeCl3 (cat) c. H2SO4 Br + HBr Cl + HCl SO3H + H2 O Friedel-Crafts H3C CH3 + + AlCl3 (cat) HCl Cl O H3C Cl 1. AlCl (cat) 3 + CH3 + Charles Friedel HCl 2. H2O O O H3C O CH3 + O O 1. AlCl3 (cat) 2. H2O CH3 + H3C O OH James Mason Crafts Homework: Give a mechanism and rationalise the substitution pattern FeCl3 Cl Mechanism for Acylation Mechanism for Alkylation Water is required here Reactions of Substituted Benzenes CH3 CH3 CH3 CH3 NO2 HNO3 / H2SO4 + + 59% 4% NO2 toluene NO2 37% alkyl groups are weakly activating at ortho- & paraToluene reacts 25 times faster than benzene in nitration NH2 OH NH2 Br2 aniline Br Note – no catalyst Br2 Br Br 100% OH phenol Br Br Br 100% -NH2, -OH, -OMe, MeCONH- are all powerful activating groups Ortho- and para- directing Alkyl groups (e.g. CH3) inductively donate electrons (+I) (through σ-bonds) CH3 +I H + H N NH2 _ +R activate through π-bonds +R Activates o & p towards E+ attack H + H N _ OH OH Br Br2 CH3 NHCOCH3 CH3 The strongest activator wins out NHCOCH3 Br2 Br Br NHCOCH3 directs para, but makes benzene less reactive compared to aniline Strong –R Deactivators: NO2, CHO, COOH, CO2H, CN Deactivates o & p towards E+ attack; Thus, strong meta-directors NO2 NO2 NO2 NO2 NO2 HNO3 / H2SO4 + + 6% 93% NO2 Undergoes nitration 10,000 times slower than benzene O + N O O + O N 1% O + O N - R, -I + + NO2 CH3 CH3 NO2 HNO3 /H2SO4 NO2 directors reinforce each other NO2 Halogens are weak deactivators – directing ortho- and paraF Cl + I Br + F F _ - I, +R Br Br Cl2, FeCl3 Br Cl + _ o-bromochlorobenzene Cl p-bromochlorobenzene Tutorial Questions HNO3 / H2SO4 NO2 HNO3 / H2SO4 A + B +C isomeric dinitrobenzenes 85% 1. Write a mechanism for the generation of the nitronium ion (NO2+) from concentrated nitric and sulfuric acid. 2. Write a mechanism for the nitration of benzene. 3. Draw dinitrobenzenes A-C. 4. Predict the ratio of isomeric products A-C from the nitration of nitrobenzene, and comment on the rate of nitration of benzene compared to nitrobenzene. Rationalize your answers with resonance structures. 5. Draw the structure of an aromatic that undergoes electrophilic aromatic substitution faster than benzene. Briefly give reasons for your answer. Tutorial Questions 1. What are the four rules of aromaticity? 2. Draw one aromatic molecule, which does not contain a benzene ring. 3. Draw the following molecules; ortho-dibromobenzene, para-nitroaniline, phenol, meta-nitroanisole and 2,4,6-trinitrotoluene (TNT) 4. Predict the major product for the following reaction, and name the product. OH + major product Br2 CH3 5. Provide a brief synthesis for TNT starting from toluene 6. Provide a mechanism for the following reaction; O AlCl3 Cl O Synthesis of Substituted Benzenes Using Arenediazonium Salts _ + N N Cl + Nu _ _ Nu + N2 + Benzenediazonium chloride NH2 NaNO2, HCl 0 ºC HNO2 Nitrous acid made on ice _ + N N Cl Cl _ + N N Cl + Nu _ _ Nu + NO2 HCl, NaNO2 _ + N N Br _ + N N Cl Br + Cl _ + N N Cl NH2 Sn / HCl N2 CN CuCN CuBr - N2 - N2 CH3 _ + N N Cl CH3 I KI - N2 CH3 CH3 Arenediazonium Ion as an Electrophile Aryl diazonium salts undergo coupling reactions with activated aromatic rings, such as phenols and anilines to yield brightly coloured azo compounds (Ar-N=N-Ar) NH2 _ + N N Cl NH2 N N + Diazonium coupling is a typical electrophilic aromatic substitution, where the phenol or aniline is the electron-rich aromatic and the diazonium salt is the electrophile. Electrophilic aromatic substitution occurs at the para-position to the electron-rich aromatic William Perkin Examples of Azo-Dyes + N2 Cl _ H3C N CH3 CH3 NaOAc, 0 ºC, H2O + N N N CH3 Yellow of margarine + N2 Cl _ OH + NaOH, 0 ºC, H2O N N OH Orange solid The mechanism for formation of an azo-dye will be described in your lectures Tutorial Questions 1. Draw the chemical structure of A, and give a full reaction mechanism for the formation of yellow azo-compound B (Scheme 1). + N2 Cl _ + A CH3 NaOAc, 0 ºC, H2O N N N CH3 B Scheme 1 NO2 HNO3 / H2SO4 NH2 Br2 / FeBr2 reagents X C A Br reagents & conditions, Y D E Scheme 2 D Br CuBr Br 2. Draw structures A, C and E, and give reagents & brief conditions X and Y. 3. Give a mechanism for the formation of C (Scheme 2). Amines Amines are derivatives of Ammonia Ammonia & amines are bases by virtue of their lone pair of electrons .. .. N H N H R H 107O CH3 H H Ammonia NH3 R R = alkyl groups 107O H N R N H H Methylamine NH2Me 1º amine CH3 CH3 N H CH3 Dimethylamine NHMe2 2º amine N CH3 CH3 Triethylamine NMe3 3º amine Amines are bases because of the lone pair on the nitrogen atom - red litmus paper to blue H H Cl NH2 Base + Acid N H Cl H Ammonium Salt = O H O O O H O oxalic acid + 2 N(CH2CH3)3 triethylamine O O + 2 HN(CH2CH3)3 O triethylaminium oxalate Aniline is a weaker base than aliphatic amines due to the resonance delocalisation of the lone pair of electrons in the free amine H N H H + H N _ This delocalisation of the lone pair of electrons makes it less available to H+ The delocalisation is lost upon formation of the salt Reactions of Amines with Nitrous Acid - Revisited 1º aliphatic amines form very unstable diazonium salts 1º aromatic amines form stable diazonium salts at 0 ºC of great synthetic utility Secondary Amines with nitrous acid Me2NH + HCl + NaNO2 N-Nitrosoamines Me N N O Me N-Nitrosodimethylamine (yellow oil) H N + HCl + NaNO2 N O N Me Me N-Nitoso-N-methylaniline (yellow oil) Tertiary Amines with nitrous acid Nitrous acid reacts to give salts with 3º aliphatic amines (not useful), with 3º aromatic amines NO+ becomes an electrophile in electrophilic aromatic substitution Me Me + HCl + NaNO2 N Me O N N Me p-Nitoso-N,N-dimethylaniline (exclusive product) Tutorial Questions 1. Draw the structure of a primary, a secondary and a tertiary aliphatic amine, and name all three amines. 2. Give a mechanism for the reaction of triethylamine with HCl. 3. Explain the origin of amine basicity and how does the basicity of aniline compare with that of a primary aliphatic amine. Draw resonance structures to explain your answer. 4. Describe the reactions of nitrous acid (HNO2) with primary, secondary and tertiary aromatic amines. There are about 20 naturally occurring amino acids 20n possible combinations of amino acids in a peptide There are eight essential amino acids All are chiral apart from glycine, R = H H CO2H H2N R All DNA encoded aa are usually L- aa are covalently linked by amide bonds (Peptide Bonds) The resulting molecules are called Peptides & Proteins R' R' N C O R N C R O Features of a Peptide Bond; 1. Usually inert 2. Planar to allow delocalisation 3. Restricted Rotation about the amide bond 4. Rotation of Groups (R and R’) attached to the amide bond is relatively free CH2OH CH3 O O H3N CH H3N C O C H3N C O O O Serine Alanine Valine - 2 H2O CH3 O H N H3N C C O CH2OH O N H C O Tripeptide : Ala . Ser. Val Strong Acid Required to hydrolyse peptide bonds Primary Structure is the order (or sequence) of amino acid residues Peptides are always written and named with the amino terminus on the left and the carboxy terminus on the right Lys. Cys. Phe 1. RSH Phe. Ser. Cys 2. 6 M HCl hydrolysis Lys + 2 Cys + 2 Phe + Ser Ph Cysteine residues create Disulfide Bridges between chains (CH2)4NH2 O H N H2 N This does not reveal Primary Structure C C OH N H O O S S Ph O H N H2 N C OH N H O C O HO C Two Nucleic Acids (Polymers) – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) Mild degradation yields monomeric units Nucleotides Complete degradation yields 1. A Heterocyclic Base Pyrine or Pyrimidine 2. A five Membered Monosaccharide 3. A Phosphate ion O O P O O D-Ribose or 2-deoxy-D-ribose The Phosphate ester can be at C-5’ or C-3’ RNA - Nucleotide Hydolysis of Phosphate Heterocyclic Base O 5' HO P N O O H2C 1' 4' OH Nucleoside N- Glycosidic linkage 2' 3' OH OH DNA - Nucleotide Heterocyclic Base O 5' HO P O O H2C N 1' 4' OH 3' 2' OH H N- Glycosidic linkage NH2 N N H (A) N H3C (C) N NH2 Guanine Pyrimidines O H H N O N H Cytosine N (G) O N H N Adenine NH2 O N N N H Purines N O H Thymine (DNA (T) N O H only) Uracil (RNA (U) only) O NH2 N N N N N HO N Nucleosides that can be obtained from DNA N HO NH2 O O H NH H H OH H H H H H OH H 2'-Deoxyadenosine H 2'-Deoxyguanosine NH2 O N O HN N HO O N HO O O H H OH H H H 2'-Deoxycytidine H H OH H H H 2'-Deoxythymidine NH 2 Adenine N N N N O 5' O O H H O O P HN H 3' H O Base Sequence is the Genetic Code Primary Structure H O N Thymine 5' O O O H H H 3' O H O P O H NH H N 5' O O N O H H O 3' H P N NH 2 Guanine O H 5' A T G 3' O Nucleotides are held together by phosphate ester linkages. Phosphate esters link 3’- OH of one ribose (or deoxyribose) with the 5’-OH of another. This makes the nucleic acid a long unbranched chain with a backbone of sugar and phosphate units with heterocyclic bases protruding from the chain at regular intervals. Secondary Structure H H3C H O N N N E. Chargaff N N H N N Adenine O Thymine H N N O H H N N N N O Cytosine N H Guanine N H Two Complementary Chains Result Watson-Crick Model of DNA (1953) Double Helix is the Secondary Structure of DNA Two nucleic acid chains are held together by weak H-bonds between bases of opposite strands Wound into a helix with a common axis The base pairs are on the inside of the helix and the sugar-phosphate backbone is on the outside 34A repeating unit contains 10 successive nucleotide pairs Phosphate-Sugar backbone is regular, base pairs can assume many different permutations Acetal Formation is REVERSIBLE and acid catalysed. Anhydrous conditions H+ R C O H + R OH R OH R OR C - H2O H OR + H2O , H O Hydrolysis Tetrahydrfuran O Tetrahydropyran HEMIACETAL FORMATION CH3 OH H H C O H3C 5-Hydroxyhexanal (6%) O C OH 2-Hydroxy-6-methyltetrahydropyran (94%) Explaining That Glucose is a Reducing Sugar CH2OH O OH O H Open form is an aldehyde OH OH D-Glucopyranose To be drawn in the lectures as a Fischer Formula (shown as a Howarth Projection) Since, aldehydes are readily oxidised, glucose is called a Reducing Sugar Sucrose is a non-reducing Sugar (cannot open up to form an aldehyde) Tutorial Questions 1. Draw the Haworth projection of glucose, and briefly explain why it is a reducing sugar. 2. Draw the DNA-encoded tripeptide, Ala.Gly.Ser. 3. Draw one DNA and one RNA nucleoside. 4. Write notes on the structure of DNA. Emil Fischer 1902 Noble Prize in Chemistry W. N. Haworth 1937 Noble Prize in Chemistry