Retinal Vein Occlusions
advertisement

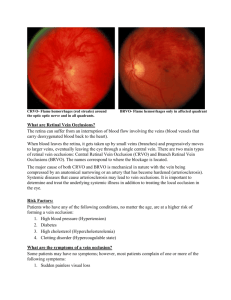
Retinal Vein Occlusions Morphology CRVO BRVO Hemispheric VO Hemicentral VO Papillophlebitis Macular BRVO CENTRAL RETINAL VEIN OCCLUSION » The actual mechanisms producing the clinical picture of central retinal vein occlusion may be roughly divided into those conditions that produce a physical blockage at the level of the lamina cribrosa, and those conditions in which hemodynamic factors result in an obstruction to the flow of blood. These mechanisms probably coexist in many patients with central VO. "Blood and thunder" appearance of a central retinal vein occlusion. PATHOLOGY Histopathologic evaluation of eyes removed because of a central retinal vein occlusion demonstrates an occlusion at or just behind the level of the lamina cribrosa. At this location, there are certain anatomic factors that predispose the central retinal vein to occlusion. First, the lumina of the central retinal artery and central retinal vein are narrower than they are in the orbital optic nerve, and the vessels are bound by a common adventitial sheath. Anatomical Studies Green studied 29 eyes that were enucleated 6 hours to 10 years after occlusion. As a result of this study, they hypothesized that the flow of blood through the central retinal vein becomes increasingly turbulent as the vein progressively narrows at the lamina cribrosa, where it also may be further impinged upon by arteriosclerosis of the adjacent central retinal artery. This turbulence damages the endothelium in the retrolaminar vein, which exposes collagen and initiates platelet aggregation and thrombosis. Their studies show the evolution of this thrombus. Initially, the thrombus adheres where the endothelium has been severely damaged. Doppler Studies Recently, color Doppler ultrasound imaging has been used to examine the blood flow in the orbit, including the optic nerve head, and has been used to examine patients with central retinal vein occlusion. As might be expected, the venous velocity in the eye of a patient with central retinal vein occlusion is markedly reduced compared either with the unaffected eye or to control eyes. There is evidence, however, that the central retinal artery blood flow is also impaired in eyes with acute central retinal vein occlusion. In addition, vascular resistance is slightly higher in the ophthalmic artery and short posterior ciliary arteries of both the involved and the clinically healthy fellow eye of patients with central retinal vein occlusion compared with control eyes. There is also a trend toward higher vascular resistance of the central retinal artery in the clinically healthy eyes of patients with central retinal vein occlusion compared with control eyes. Risk Factors An increased risk of central retinal vein occlusion was found in patients with systemic hypertension, diabetes mellitus, and open-angle glaucoma; the risk of central vein occlusion was decreased for patients with increasing levels of physical activity and increasing levels of alcohol consumption. For women, the risk decreased with the use of postmenopausal estrogen and increased with a higher erythrocyte sedimentation rate. The Eye Disease Case-Control Study Group: Risk factors for central retinal vein occlusion. Arch Ophthalmol 114:545, 1996 Risk Factors for Central Retinal Vein Occlusion Investigations All patients with central retinal vein occlusion should have a comprehensive ophthalmic evaluation, including an appropriate evaluation for glaucoma. In addition, they should be referred to their primary care physician for an evaluation of cardiovascular risk factors, including hypertension and diabetes GENERAL PRINCIPALS Maximise Prevent Recovery and Vision re-occlusion Detect associated systemic disease Detect / Prevent Glaucoma Protect other eye Standard Investigations FBC, PV, ESR U+E, Creatinine LFT, Protein Electrophoreseis Random Glucose, Lipid Urine analysis Ophthalmic Investigations FFA CDI (Color doppler ) Carotid disease-Using digital subtraction angiography, Brown and associates studied 37 patients with central retinal vein occlusion; they found that significant ipsilateral stenosis (greater than 50%) was not higher in these patients compared with historically matched controls. They did find, however, that patients with ischemic central retinal vein occlusion had a higher incidence of overall carotid atherosclerotic obstruction (ipsilateral and contralateral) than patients with nonischemic central retinal vein occlusion Thrombophilic Screen ( less than 50 years ) Clotting screen Protein C,S defficiency Elevated factor V Actviated protein C resistance Factor V Leiden a major risk factor in females (Five percent of European population) Dysfibrogenaemia (1/3000) Prothrombin G20210A Antiphopholipid antibodies Ischemic Central Retinal Vein Occlusion Patients with an ischemic pattern are usually aware of a sudden, painless decrease in visual acuity. Vision ranges from 20/400 to hand movements. The onset, however, is generally not as rapid or the visual loss as extensive as in central retinal artery occlusion. Exceptional cases have been noted in which patients with an acute onset had reasonably good vision and yet demonstrated a picture of ischemic central retinal vein occlusion. Patients with ischemic occlusion have an average age of 68.5 years. Nonischemic Central Retinal Vein Occlusion Nonischemic central retinal vein occlusion is a much milder and more variable disease in appearance, symptoms, and course compared with ischemic central retinal vein occlusion. Patients with nonischemic central retinal vein occlusion are an average of 5 years younger (average age, 63 years) than those with ischemic vein occlusion Ophthalmoscopic features Confluent hemorrhages are the most prominent ophthalmoscopic feature of an acute ischemic central retinal vein occlusion These hemorrhages occur in a wide variety of shapes and sizes; they are usually concentrated in the posterior pole, but may be seen throughout the retina. Hemorrhages in the superficial retina may be so prominent about the posterior pole that the underlying retina is obscured. Many hemorrhages are flame shaped, reflecting the orientation of the nerve fibers. Dot and punctate hemorrhages are interspersed and indicate involvement of the deeper retinal layers. Bleeding may be extensive, erupting through the internal limiting membrane to form a preretinal hemorrhage or extending into the vitreous. Small dot hemorrhages may be seen either isolated or clustered around small venules. The entire venous tree is tortuous, engorged, dilated, and dark. The retina is edematous, particularly in the posterior pole; some of this edema may obscure portions of the retinal vessels. Cotton-wool patches (soft exudates) are often present. The disc margin is blurred or obscured, and the precapillary arterioles appear engorged. Splinter hemorrhages and edema are present on the disc surface and extend into the surrounding retina. The physiologic cup is filled, and the venous pulse is absent. The arterioles, often overlooked because of the other more striking pathologic features, are frequently narrowed. Sometimes in central retinal vein occlusion of acute onset, the fundus picture is less dramatic, and all of the findings previously discussed may be present, but to a lesser degree. Vision depends on extent of macular involvement. Angiography The intravenous fluorescein angiogram pattern of an ischemic central retinal vein occlusion is usually characterized by a delayed filling time of the venous tree of the retina, capillary and venous dilation, and extensive leaking of fluorescein into the retina, particularly in the macular area and in the area adjacent to the larger venous trunks and capillary nonperfusion may not be noted at the time of initial occlusion, but are usually manifest shortly thereafter. Late-phase photographs show patchy extravascular areas of fluorescence and staining of the retinal veins. The intravenous fluorescein angiogram pattern of an ischemic central retinal vein occlusion is usually characterized by a delayed filling time of the venous tree of the retina, capillary and venous dilation, and extensive leaking of fluorescein into the retina, particularly in the macular area and in the area adjacent to the larger venous trunks and capillary nonperfusion Microaneurysms may not be noted at the time of initial occlusion, but are usually manifest shortly thereafter. Late-phase photographs show patchy extravascular areas of fluorescence and staining of the retinal veins. Fluorescence in the macula indicates capillary leakage and edema; this not only may account for much of the initial visual loss in the acute phase, but may eventually result in permanent structural changes. Classifying ischaemia The amount of nonperfusion or ischemia is determined by inspecting the fluorescein angiography negative under magnification. The photographer inspects not only the central 30° or 45°, but as much of the peripheral retina as possible. Another method has been to classify eyes with less than 10 disc diameters of perfusion on fluorescein angiography as perfused or nonischemic, and eyes with 10 or more areas of nonperfusion as nonperfused or ischemic. Macular Oedema Fluorescence in the macula indicates capillary leakage and edema; this not only may account for much of the initial visual loss in the acute phase, but may eventually result in permanent structural changes. Prognosis CRVO The prognosis for ischemic central retinal vein occlusion is generally poor because of decreased visual acuity and neovascularization. Visual loss occurs because of macular edema, capillary nonperfusion, overlying hemorrhage (either retinal or vitreal), or a combination of all of these. Retinal edema usually gradually subsides except in the macula, where it may persist for many months or years. Macular holes or cysts may form. Neovascularization The most serious complication of central retinal vein occlusion is neovascularization. Neovascularization elsewhere (NVE) occurs less frequently than neovascularization of the iris (NVI), and usually only in ischemic occlusions. The low incidence of retinal surface neovascularization in ischemic central retinal vein occlusion is thought to be due to the destruction of endothelial cells, which provide the source for endothelial proliferation and neovascularization. Percentage of Ocular Neovascularization in Venous Occlusion Neovascularization of the Iris. Neovascularization of the iris and frequently neovascular glaucoma occurs in approximately 8%6to 25% of all central retinal vein occlusions and generally only in those eyes that exhibit an ischemic pattern of occlusion. Magargal and co-workers have shown that the incidence of neovascularization increases dramatically above approximately 50% capillary nonperfusion. The incidence of anterior segment neovascularization in nonischemic central retinal vein occlusion is approximately 1%, compared with approximately 35% to 45% for ischemic central retinal vein occlusion. Neovascularization of the iris or angle is significantly correlated with the extent of capillary nonperfusion on the fluorescein angiogram. Rubeosis developed in 80% to 86% of the eyes with severe nonperfusion of three to four quadrants of the posterior pole or the periphery, but in only 3% to 9% of those with less capillary nonperfusion. Neovascularization of the Iris Neovascularization of the iris may develop as early as 2 weeks after central retinal vein occlusion or as late as 2½1/2 years Neovascularization of the iris will develop in almost all patients within the first year, but usually in the first 3 months.89 Symptomatically, patients complain of tearing, irritation, pain, and further blurring of vision as the intraocular pressure in the affected eye begins to rise. The pain may become excruciating. The cornea is hazy and the pupil dilated, and a network of fine vessels is seen over the surface of the iris (rubeosis iridis) on slit-lamp examination. By the time gonioscopy reveals extension of this neovascular membrane into the trabecular network and throughout the angle, the intraocular pressure is usually markedly elevated. The angle is initially open, but later in the disease, peripheral anterior synechiae develop and the angle may become irreversibly closed, resulting in neovascular glaucoma. Large, extremely irritating bullae may form on the surface of the cornea and then break down. Dense cataracts eventually form, obscuring the fundus. HEMICENTRAL AND HEMISPHERIC RETINAL VEIN OCCLUSION The terms hemicentral retinal vein occlusion and hemispheric retinal vein occlusion refer to eyes in which approximately half of the venous outflow from the retina, either the superior or the inferior, has been occluded. In approximately 20% of eyes, the branch retinal veins draining the superior and inferior halves of the retina enter the lamina cribrosa separately before joining to form a single central retinal vein. Hemicentral retinal vein occlusion is an occlusion of one of these dual trunks of the central retinal vein within the nerve. Hemispheric retinal vein occlusion is an occlusion involving the venous drainage from approximately half of the retina, either the superior or the inferior retina Hemispheric retinal vein occlusions In some eyes, the nasal retina is not drained by a separate vein, but by a branch of either the superior or the inferior temporal vein. It is the occlusion of one of these veins draining both the nasal retina and the superior or inferior retina near the optic disc that accounts for the majority of hemispheric retinal vein occlusions. The treatment and classification are similar to that of branch retinal vein occlusion. BRANCH RETINAL VEIN OCCLUSION PATHOLOGY Leber was probably the first investigator to note the connection between branch retinal vein occlusion and the arteriovenous intersection. Koyanagi found that the majority (77.7%) of his cases of temporal vein occlusion involved the superior retina. He attributed this to the preponderance of arteriovenous crossings in this region compared with other quadrants.Others later confirmed this anatomic observation, noting that branch retinal vein occlusion always occurs at an arteriovenous intersection.Both fluorescein angiography1and histopathologic examination confirm that most occlusions occur at an arteriovenous crossing and that the few that do not are in the vicinity of a retinal artery. Histologically, where the vein and artery cross, they share a common adventitial sheath, and the venous lumen may be diminished by as much as a third at this crossing. Morphology The clinical picture of branch retinal vein occlusion is retinal hemorrhages that are segmental in distribution. The apex of the obstructed tributary vein almost always lies at an arteriovenous crossing. Usually some degree of pathologic arteriovenous nicking is present. The occlusion is commonly located one or two disc diameters away from the optic disc. However, the occlusion may lie at a point near the disc edge or, less frequently, may involve one of the smaller, more peripheral tertiary or macular branches. Risk Factors for Branch Retinal Vein Occlusion Systemic hypertension History of cardiovascular disease Increased body mass index at 20 years of age cholesterol History of glaucoma High serum levels of a2-globulin Management of BRVO Branch vein obstruction is often associated with pre-existing vascular disease. Evaluation for systemic abnormalities, in particular hypertension, should be performed. Exclusion of diabetes, hyperlipidaemia, hyperviscosity/coagulation states, antiphospholipid syndrome, or any other predisposing condition should be performed. Regular review is required until the haemorrhages clear so that the most suitable treatment option can be achieved. Approximately one third to one half of patients with BRVO have recovery of visual acuity to 20/40, or better, without therapy. An important complication of branch retinal vein occlusion is neovascularization Neovascularization of the iris and neovascular glaucoma are uncommon and occur in only approximately 1% of affected eyes. More commonly, neovascularization of the disc occurs in approximately 10% of eyes, and neovascularization elsewhere occurs in approximately 20% of eyes. Generally, retinal neovascularization occurs within the retinal area served by the occluded vessel, but it has been reported to occur outside in presumably normal retina. Vitreous hemorrhage due to neovascularization occurs in approximately half of the eyes with neovascularization.Butner and McPherson239 found that 11.3% of spontaneous vitreous hemorrhages were due to a branch retinal vein occlusion, an incidence second only to proliferative diabetic retinopathy as a cause of vitreous hemorrhage. Oyakawa and co-workers found that in 38.3% of eyes undergoing a vitrectomy for a nondiabetic vitreous hemorrhage, the hemorrhaging was due to a branch retinal vein occlusion. Branch Vein Occlusion Study Group –Vitreous Hemorrhage Of patients with ischemic vein occlusion who were treated before neovascularization occurred, 12% developed a subsequent vitreous hemorrhage, whereas only 9% of ischemic eyes treated after neovascularization occurred developed a vitreous hemorrhage. Although the study was not designed to determine the optimal time for treatment, the data suggest (but do not prove) that there may be no advantage to treatment before the development of neovascularization. The study was not able to draw conclusions about the effect of photocoagulation on the prevention of visual loss. Branch Vein Occlusion Study Group- Macular Oedema Can photocoagulation improve visual acuity in eyes with macular edema reducing vision to 20/40 or worse? Eyes with branch vein occlusion occurring 3 to 18 months earlier with 20/40 vision or worse because of macular edema (but not hemorrhage in the fovea or foveal capillary nonperfusion) were treated with the argon laser in a "grid" pattern in the area of capillary leakage. The treatment did not extend closer to the fovea than the avascular zone and did not extend outside the peripheral arcade. At the 3-year follow-up, there was a statistically significant improvement in the visual acuity of treated eyes compared with untreated eyes. MACULAR BRANCH RETINAL VEIN OCCLUSION An occlusion limited to a small venous tributary draining a section of the macula and located between the superior and inferior temporal arcades is considered a subgroup of branch retinal vein occlusion.Most patients with macular branch vein occlusion complain of blurring or distortion of vision. Superior macular vein occlusions are more common than inferior macular vein occlusions, and some degree of macular edema is present in approximately 85% of these eyes. Although small areas of capillary nonperfusion are present in approximately 20% of eyes, neovascularization is not seen. This type of macular vein occlusion can be remarkably subtle at times. Joffe and associates pointed out that clues such as small collateral channels and microaneurysms often suggest the diagnosis. Treatment of macular edema in macular vein occlusion by photocoagulation is identical to the treatment of other branch retinal vein occlusion. Macular Oedema- FFA PAPILLOPHLEBITIS In 1961, Lyle and Wybar described six young, healthy patients with a unilateral, relatively benign condition characterized by mild blurring of vision, essentially normal visual acuity, dilated and tortuous retinal vessels, a varying amount of retinal hemorrhage, and optic disc edema All six patients improved spontaneously, but were left with sheathing of retinal vessels and the formation of vessels on the optic disc. Lyle and Wybar called this condition "retinal vasculitis" and believed it to be due to a central retinal vein occlusion secondary to an inflammatory vasculitis of the venous system. Lonn and Hoyt agreed with this etiology, but felt that "papillophlebitis" was a more appropriate descriptive term. Hart and co-workers, however, pointed out that an inflammatory etiology for this disease is tenuous, and no well-documented cases have been studied histopathologically. Investigations and therapy GENERAL PRINCIPALS Maximise Recovery and Vision Prevent re-occlusion Detect any associated systemic disease Detect / Prevent Glaucoma Protect other eye General Therapy Avoid oral contraceptives Aspirin Treat hypercholesterolemia and hypertension Lower IOP Anticoagulants if required If vision drops consider re-occlusion. Panretinal photocoagulationSummary Panretinal photocoagulation has been recommended for the treatment of neovascularisation secondary to CRVO's. There is currently debate regarding the timing of this therapy. Whether delayed intervention (after the development of iris new vessels) offers as good an outcome as early laser treatment(at the time of neovascularisation of the retina alone) needs to be shown. Grid therapy for macular oedema in CRVO has not been shown to improve visual acuity. Central Retinal Vein Occlusion Study Group - Photocoagulation Hayreh and associates conducted a prospective but nonrandomized study of panretinal photocoagulation in ischemic central retinal vein occlusion. They found no statistically significant difference between the treated and untreated groups in the incidence of angle neovascularization, neovascular glaucoma, retinal or optic nerve neovascularization, vitreous hemorrhage, or visual acuity. The only significant finding was that fewer patients in the treated group had neovascularization of the iris compared with nontreated controls, but only if the panretinal photocoagulation was applied within the first 3 months after the onset of central retinal vein occlusion and panretinal photocoagulation resulted in a significant loss of the peripheral field. Once neovascularization in the anterior segment is detected, panretinal photocoagulation should be instituted promptly. This will often result in regression of the iris vessels and prevent complete angle closure; this is also true in patients with some increase in intraocular pressure but in whom the angle is not occluded for 360°. Central Retinal Vein Occlusion Study Group- Macular Oedema The Central Retinal Vein Occlusion Study Group performed a randomized, prospective clinical trial on the effect of macular grid photocoagulation compared with no treatment on eyes with 20/50 or worse visual acuity due to macular edema with no capillary nonperfusion on fluorescein angiography. Although grid photocoagulation lessens macular edema both angiographically and clinically, there was no difference in visual acuity between the treated and untreated patients. For treated patients, there was a trend toward decreased visual acuity in patients older than 60 years and visual improvement in patients younger than this; this effect was not seen in untreated patients. Although this study suggests a possible benefit to visual acuity in younger patients with macular edema who are treated compared with untreated controls, the number of patients in this subgroup is too small for a statistically valid comparison of treated versus untreated eyes. Chorioretinal anastomosis in patients with nonischemic central retinal vein occlusion. McAllister and Constablereported a surgical technique to create a chorioretinal anastomosis in patients with nonischemic central retinal vein occlusion. Their current technique is to rupture Bruch's membrane first in an area adjacent to the edge of a vein located at least three disc diameters from the optic disc with the argon laser; they then use a YAG laser to create a small opening in the sidewall of the adjacent vein. In their study there was an average of 2.1 attempts to create an anastomosis, which was successful in only 42% of the patients in the first series171 and 67% of patients in the second series.172 In the first series, ischemic central vein occlusion did not develop in any of the patients in whom a successful anastomosis was produced, but it did develop in 31% of patients in whom such an anastomosis could not be created.171 It should be noted, however, that this is not a control group, and they have not reported on a controlled clinical trial of this procedure. All the patients with a successful anastomosis had an improvement in final visual acuity compared with pretreatment visual acuity. In the group of patients with an unsuccessful anastomosis, 38% had an improvement in visual acuity, 44% had a worse visual acuity, and 19% had no change. There were some minor complications, such as vitreous and retinal hemorrhages, that tended to clear fairly well. However, there were some major complications, including a major fibrovascular proliferation at 14% of the sites where surgery was attempted.This complication can lead to serious, nonclearing vitreous hemorrhages and/or traction retinal detachment and may require a vitrectomy for treatment. Lacking a controlled clinical trial for this new treatment, there is no way to know whether laser chorioretinal anastomosis is more effective for nonischemic central retinal vein than no treatment. Neovascular Glaucoma Once developed, neovascular glaucoma responds poorly to any type of treatment. Cycloplegics, topical pressure-lowering agents, carbonic anhydrase inhibitors, and corticosteroids, though failing to lower the intraocular pressure significantly, may make the patient more comfortable. Panretinal photocoagulation often cannot be applied in cases of advanced neovascular glaucoma in which the angle has been substantially occluded and the cornea may be too cloudy to allow treatment. Trans-scleral cyclocryotherapy or trans-scleral laser cyclodestruction, sometimes combined with 360° of trans-scleral panretinal cryoablation,has also been used to preserve the globe. In some cases where visibility is poor and the angle is closed, we have had some success in the last few years combining pars plana vitrectomy and endophotocoagulation with a drainage implant Contact Us Author : John G. O'Shea MD Illustrations: Robert Harvey FRCSEd (from Practical Ophthalmology, 2002 Palmtrees Publishing) Rob Harvey E-mail Address : rob_harvey@msn.com Correspondence Birmingham and Midland Eye Centre, Dudley Rd, Birmingham B18 7QH, U.K