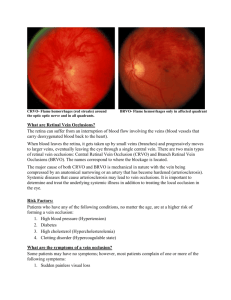
Ranibizumab for Retinal Vein Occlusion
advertisement

Ranibizumab for the treatment of macular oedema secondary to retinal vein occlusion (RVO), NICE TA283, May 2013 Only fully completed forms will be accepted for consideration The completed form must be sent by the hospital commissioning team to the High Cost Drugs Team at hvccgdrugrequests@nhs.net If the patient does not fulfil NICE criteria The responsible commissioner will not normally fund any treatment where the patient does not meet the agreed criteria as outlined in this patient specific funding application form. Following a clinical trial, the responsibility for ongoing funding remains with the provider or pharmaceutical company. The commissioner will only fund treatment that meets the commissioned pathway. Applications can be made via the Individual Funding Requests process ONLY where the patient has exceptional clinical circumstances. Please check the commissioner websites for contact details of the IFR team. Payment by the commissioner will only be made if the completed form is received no later than 15 days after INITIAL treatment commences Patient NHS No. Trust: GP Name: Patient Hospital Number: Consultant Making Request: Patient initials: Patient Dob: GP code / Practice code: / / GP Post code: Criteria for initiation Indicate form of RVO CRVO Which eye is to be treated? Left eye What is the visual acuity at baseline (Snellen score)*? Left eye BRVO Right eye Right eye Date measured In line with NICE, commissioners would expect treatment will usually be administered to worse seeing eye (unless no vision in one eye) Further Initiation Criteria specific for CRVO/BRVO Central Retinal Vein Occlusion (CRVO) Branch Retinal Vein Occlusion (BRVO) OCT> 250microns Yes/ No OCT > 250microns Laser coagulation has been tried and was unsuccessful, or the extent of the disease makes laser coagulation unsuitable Please confirm that the treatment activity is charged as BZ23Z Vitreous retinal procedures cat 1, out-patient procedure (see Costing Template for TA 283). Only outpatient activity will be funded. Pharmacy and Medicines Optimisation Team Herts Valleys Clinical Commissioning Group (HVCCG) Yes/ Yes/ No No Yes/ No Yes The treatment will be discontinued if there is no significant improvement in visual acuity over the course of the first three injections, in line with the Summary of Product Characteristics (SPC) Clinician’s Declaration Yes I confirm that I have discussed with the patient and that they understand and consent to their personal information being shared with commissioning support organisations. I have also recorded this discussion in the patient’s notes. I confirm the risks and benefits of treatment have been fully discussed with the patient and documented. I confirm that funding approval is subject to initiation and follow up of treatment response being undertaken by a specialist ophthalmology team. I acknowledge and adhere to the cost effective use of this treatment as advocated in NICE TA 283 and believe that within this Trust the above patient would be best managed using the treatment as requested above. If this patient is being jointly managed by a second consultant, please Name of consultant (or clinician delegated by consultant): state name here: Name: Signature (electronic signature): Date: Date: / / Signature (or email confirmation) by Trust Chief Pharmacist (or nominated deputy) Name: Signature: Date: / / Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion, NICE TA 283: 1.1 Ranibizumab is recommended as an option for treating visual impairment caused by macular oedema: following central retinal vein occlusion or following branch retinal vein occlusion only if treatment with laser photocoagulation has not been beneficial, or when laser photocoagulation is not suitable because of the extent of macular haemorrhage and only if the manufacturer provides ranibizumab with the discount agreed in the patient access scheme revised in the context of NICE TA274. COMMISSIONING ARRANGEMENTS FOR RANIBIZUMAB FOR DIABETIC MACULAR OEDEMA SECONDARY TO RETINAL VEIN OCCLUSION 1. Ranibizumab for visual impairment due to retinal vein occlusion will only be funded under the NHS in line with NICE TA 283. 2. Visual acuity in the treated eye must be between 6/12 and 6/96 and OCT>250micron, (based on Royal College of Ophthalmologists Interim Guidelines for Management of Retinal Vein Occlusion Dec 2010 and in line with inclusion criteria in BRAVO and CRUISE).* 3. Treatment is given monthly and continued until maximum visual acuity is achieved i.e the patient's visual acuity is stable for three consecutive monthly assessment performed while on ranibizumab treatment. If there is no improvement in visual acuity over the course of the first three injections, continued treatment is not recommended. 4. Thereafter patients should be monitored monthly for visual acuity. 5. Treatment is resumed when monitoring indicates loss of visual acuity due to macular oedema secondary to RVO. Monthly injections should then be administered until stable visual acuity is reached again for three consecutive monthly assessments (implying a minimum of two injections). The interval between two doses should not be shorter than 1 month. 6. If treatments are not provided in line with the criteria laid out in this document, HVCCG reserves the right to re-claim money from providers for the relevant drug and activity costs. HVCCG will request an audit around patient outcomes on an annual basis. Pharmacy and Medicines Optimisation Team Herts Valleys Clinical Commissioning Group (HVCCG)