Blood Coagulation
advertisement

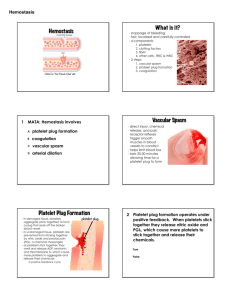
BLOOD COAGULATION week 7-8 Platelets Platelets are fragments of megakaryocytes with a blue-staining outer region and a purple granular center Their granules contain serotonin, Ca2+, enzymes, ADP, and platelet-derived growth factor (PDGF) Platelets function in the clotting mechanism by forming a temporary plug that helps seal breaks in blood vessels Platelets not involved in clotting are kept inactive by NO and prostaglandin I2 Size: 1.5-3 M Life span 7-10 day Count: 150-400 x 109/L No nucleus Surface binding sites for fibrinogen, VWF Surface platelet antigens, HPA1 Functions: Hemostatic plug formation Release and synthesis of coagulation factors Platelet Formation Endomitotic synchronous nuclear replication Thrombopoietin major regulator 332 residues, Mr 38KDa C-MPL receptor for TPO on platelet 7-10 days delay to rise platelet level by TPO endomytotic synchronous nuclear replication - enlarge cytoplasmic volume - Increase of nuclear lobe ~4000 platelets from one megakaryocyte ultrastructure of platelet Hemostasis A series of reactions designed for stoppage of bleeding Hemostasis three phases occur in rapid sequence immediate vasoconstriction in response to injury Platelet plug formation Coagulation (blood clotting) Vasoconstriction and Platelet Plug Formation platelet adhesion and platelet plug formation platelets do not stick to each other or to the endothelial lining of blood vessels blood vessel wall (endothelial cells) prevent platelet adhesion & aggregation platelets contain receptors for fibrinogen and vWF after vessel injury platelets adhere and aggregate damage to blood vessel endothelium exposes collagen platelets adhere to collagen with VWF and stimulated by thromboxane A2 platelets stick to exposed collagen fibers and form a platelet plug platelets release serotonin and ADP, which attract still more platelets The platelet plug is limited to the immediate area of injury by PGI2 platelet adhesion and aggregation Integrin 21 Atherosclerotic Plaque Development The normal arterial wall advance stage of atherosclerosis, consists of smooth calcified scar tissue forms, if the muscle and connective endothelium is damaged and collagen tissue with an endothelial exposed, platelet aggregates and cell lining thrombus forms, if blood flow is stopped, heart attack ! Early stages excess LDL-cholesterol accumulates between the endothelium and connective tissue. There it is oxidized and phagocytosed. Macrophages produce paracrines that attract smooth muscle cells. fibrous plaque/ Further cholesterol accumulation results in fibrous scar tissue formation. Migrating smooth muscle cells divide, thickening the arterial wall and narrowing the lumen. Coagulation Phase Two major pathways Intrinsic pathway Extrinsic pathway Both converge at a common point reactions take place in 3 major phases: Prothrombin activator is formed Prothrombin is converted into thrombin Thrombin catalyzes the joining of fibrinogen into a fibrin mesh 13 soluble factors are involved in clotting some of these factors are dependent on Vitamin K most of these factors are proteases normally inactive and sequentially activated hereditary lack of clotting factors lead to diseases such as hemophilia -A PHASE ONE: Pathways to Prothrombin Activator initiated by either the intrinsic or extrinsic pathway triggered by tissue-damaging events involves a series of procoagulants each pathway cascades toward factor X activated factor X makes complexes with Ca++, PF3, and factor V to form prothrombin activator PHASE TWO: Pathway to Thrombin Prothrombin activator catalyzes the transformation of prothrombin to the active enzyme thrombin PHASE THREE: Common Pathways to Fibrin Mesh Thrombin catalyzes the polymerization of fibrinogen into fibrin Insoluble fibrin strands form the structural basis of a clot Fibrin causes plasma to become a gel-like trap Fibrin in the presence of Ca++ activates factor XIII that: Cross-links fibrin Strengthens and stabilizes the clot Intrinsic Pathway All clotting factors are within the blood vessels Clotting is slower Activated partial thromboplastin test (aPTT) Extrinsic Pathway Initiating factor is outside the blood vessels - tissue factor Clotting is faster - in Sec Prothrombin test (PT) Intrinsic pathway Extrinsic pathway Factors affected By Heparin Vitamin K dependent Factors affected by Oral anticoagulants Cross linked Fibrin polymer Summery Coagulation Extrinsic Factor VII Intrinsic Factors XII, XI, IX, VIII Common Path (TT) Factor X Factor Xa Fibrinogen Thrombin Fibrin Monomer Prothrombin Thrombin Fibrin Polymer F-XIIIa Fibrinogen Fibrin Cross Linked Fibrin Inhibition of Clotting Factors Fibrin acts as an anticoagulant by binding thrombin and preventing its: Positive feedback effects of coagulation Ability to speed up the production of prothrombin activator via factor V Acceleration of the intrinsic pathway by activating platelets Thrombin not absorbed to fibrin is inactivated by antithrombin III FDP – Inhibit thrombin Other Plasma Inhibitors of activated factors Heparin, 2 Antiplasmin Protein C – Factor VIII and factor V (fibrinolysis) Blood flow – dilution and clearing Plasminogen Plasmin (Fibrin, factor V and VIII) Fibrinolysis Enhance degradation of clots Activation of endogenous protease Plasminogen (inactive form) is converted to Plasmin (active form) Plasmin breaks down fibrin clots Exogenously administered drugs Streptokinase - bacterial product - continuous use immune reaction Urokinase - human tissue derived - no immune response Tissue plasminogen activator (tPA) - genetically cloned no immune reaction - EXPENSIVE Inhibitors of fibrinolysis - aminocaproic acid Lysine analog- inhibits proteases Fibrin is a fibrillar protein that is polymerised to form a "mesh" that forms a hemostatic plug or clot and is made from its zymogen FIBRINOGEN (also called factor I), a soluble plasma glycoprotein (340 kDa) synthesized in hepatocytes and megakaryocytes. Processes in the coagulation cascade activate the zymogen prothrombin to the serine protease thrombin, which is responsible for converting fibrinogen into fibrin. Fibrin is then cross linked by factor XIII to form a clot. Fibrinogen concentration in blood plasma: 1.5 - 4.0 g/L In its natural form, fibrinogen bridges between platelets, by binding to their GpIIb/IIIa membrane proteins Fibrinogen is a symmetrical dimer composed of 6 paired polypeptide chains (α, β, γ) linked by disulfide bonds N-terminal part of these 3 chains contain the Cys that participate in the crosslinking of the chains. However, there is no similarity between the C-terminal part of the α chain and that of the β and γ chains. The C-terminal part of the β and γ chains forms a domain of about 270 amino-acid residues. FIBRINOPEPTIDE a small part on the α and β chains prevent fibrinogen spontaneous self-polymer formation. Factor III (Tissue factor, thromboplastin or CD142) a protein present in subendothelial tissue, platelets, and leukocytes necessary for the initiation of thrombin formation from the zymogen prothrombin The gene is located on chromosome 1p22-p21. The protein structure of TF consists of three domains: 1. extracellular domain binds factor VIIa. One of domains of Factor VIIa is carboxylated GLA domain, binds in the presence of Ca+2 to negatively charged phospholipids. Binding of VIIa to negatively charged phospholipids greatly enhances the proteinprotein binding of VIIa to TF. 2. a domain which crosses the hydrophobic membrane. 3. cytoplasmic domain of 21 amino acids length involved in the signaling function of TF. Factor V (rarely as proaccelerin or labile factor) Unlike other coagulation factors, it is not enzymatically active but functions as a cofactor of the thrombinase complex. circulates in plasma as a single-chain molecule with a plasma half-life of about 12 hours (max 36 hours). Deficiency leads to predisposition for hemorrhage, while some mutations (most notably factor V Leiden) predispose for thrombosis The gene is located on the first chromosome (1q23) The gene spans 70 kB, consists of 25 exons, and the resulting protein has a Mr ~330000. It is genomically related to the family of multicopper oxidases, and is homologous to coagulation factor VIII. Intrinsic pathway Extrinsic pathway Factors affected By Heparin Vitamin K dependent Factors affected by Oral anticoagulants Cross linked Fibrin polymer Factor V is activated by thrombin and is able to bind to activated platelets. On activation, factor V is spliced in to heavy (Mr ~ 110K) and light chains (Mr ~ 73K) which are nonconvalently bound to each other by Ca++. The Xa requires Ca++ and Va to convert prothrombin to thrombin on the cell surface membrane that is the part of the common pathway in the coagulation cascade. Factor Va is degraded by activated protein C, one of the principal physiological inhibitors of coagulation. Protein C itself is activated by thrombin. Concentration and action of protein C are important determinants in the negative feedback loop through which thrombin limits its own activation. Factor VII (proconvertin) It is an enzyme of the serine protease class. The action of the factor is impeded by tissue factor pathway inhibitor (TFPI), which is released almost immediately after initiation of coagulation. Factor VII is vitamin K dependent It is produced in the liver Use of warfarin or similar anticoagulants impairs its function. The gene is located on chromosome 13 (13q34). Hageman factor (factor XII) It activates factor XI and prekallikrein. It is an enzyme of the serine protease (or serine endopeptidase) class Discovery (1955): from blood sample of the 37-year-old railroad brakeman John Hageman was found to have prolonged clotting time in test tubes, even though he had no hemorrhagic symptoms. Dr. Oscar Ratnoff found that Mr. Hageman lacked a previously unidentified clotting factor. Exposure of human plasma to glass, kaolin, celite, or other negatively charged surfaces initiates contact activation reactions that trigger the intrinsic coagulation pathway, the kinin-forming pathway, and the fibrinolytic pathway. Proteins that are known to be involved in contact activation of human plasma include Hageman factor, plasma prekallikrein (Fletcher factor), coagulation Factor XI (plasma thromboplastin antecedent), and high Mr kininogen. Factor IX (Christmas factor) plasma thromboplastin component (PTC) a single-chain plasma glycoprotein (Mr 57K) of Ser protease inactive unless activated by XIa (of the contact pathway) or VIIa (of the tissue factor pathway). IXa hydrolyse one Arg-Ile bond in factor X to form factor Xa. It requires Ca++, membrane phospholipids, and factor VIII as cofactors. gene is located on the X chromosome (Xq27.1-q27.2). Deficiency of factor iX causes Christmas disease (hemophilia B). Over 100 mutations of factor IX have been described; some cause no symptoms, but many lead to a significant bleeding disorder. Factors Limiting Clot Growth or Formation Two homeostatic mechanisms prevent clots from becoming large Swift removal of clotting factors Inhibition of activated clotting factors Factors Preventing Undesirable Clotting Unnecessary clotting is prevented by the structural and molecular characteristics of endothelial cells lining the blood vessels Platelet adhesion is prevented by: The smooth endothelial lining of blood vessels Heparin and PGI2 secreted by endothelial cells Vitamin E quinone, a potent anticoagulant Clot Retraction and Repair retraction – stabilization of the clot by squeezing serum from the fibrin strands Repair Clot Platelet-derived growth factor (PDGF) stimulates rebuilding of blood vessel wall Fibroblasts form a connective tissue patch Stimulated by vascular endothelial growth factor (VEGF), endothelial cells multiply and restore the endothelial lining Hemostasis Disorders: Thromboembolytic Conditions Thrombus – a clot that develops and persists in an unbroken blood vessel Thrombi can block circulation, resulting in tissue death Coronary thrombosis – thrombus in blood vessel of the heart Embolus – a thrombus freely floating in the blood stream Pulmonary emboli can impair the ability of the body to obtain oxygen Cerebral emboli can cause strokes A blood clot or thrombus is the final product of the blood coagulation steps in hemostasis. It is achieved via the aggregation of platelets that form a platelet plug, and the activation of the clotting factors. thrombus is physiologic in injury but pathologic in thrombosis. a thrombus is a blood clot in an intact blood vessel thrombus in a large blood vessel will decrease blood flow through that vessel. In a small blood vessel, blood flow may be completely cut-off resulting in the death of tissue supplied by that vessel. Thrombus dislodge from arteries and veins and become an embolus (free-floating). Venous emboli can block arterioles in the lung and pulmonary circulation Greek Thrombos meaning "lump". Thrombosis Arterial Thrombosis : Adherence of platelets to arterial walls - White in color - Often associated with MI, stroke and ischemia Venous Thrombosis : Develops in areas of stagnated blood flow (deep vein thrombosis), Red in color- Associated with Congestive Heart Failure, Cancer, Surgery. Hemostasis Disorders: Thrombocytopenia condition where the number of circulating platelets is deficient Patients show petechiae (small purple blotches on the skin) due to spontaneous, widespread hemorrhage Caused by suppression or destruction of bone marrow (e.g., malignancy, radiation) Platelet counts less than 50,000/mm3 is diagnostic for this condition Treated with whole blood transfusions Disseminated Intravascular Coagulation (DIC) or consumptive coagulopathy a pathological process where the blood starts to coagulate throughout the whole body resulting in depletion of platelets and coagulation factors, paradoxically increases the risk of hemorrhage. CAUSES of DIC Sepsis, particularly with gram-negative bacteria. Obstetric complications (most common), chemicals from the uterus being released into the blood, or from amniotic fluid embolisms, and eclampsia or abruptio placentae. Tissue trauma such as burns, accidents, surgery or shock. Incompatible blood transfusion reactions or massive blood transfusion Liver diseases, malignant cancers (Acute promyelocytic leukemia) or hypersensitivity reactions can produce chemicals leading to DIC. Viral hemorrhagic fevers bring about their frank effects, paradoxically, by causing DIC. Envenomation by some venomous snakes such as the Stephens Banded Snake, Hoplocephalus stephensi Hemostasis Disorders: Hemophilias hereditary bleeding disorders caused by lack of clotting factors Hemophilia A: common type (83%) deficiency of factor VIII Hemophilia B: a deficiency of factor IX Hemophilia C: mild type, a deficiency of factor XI Who can get hemophilia? 1 in 5,000-10,000 male births have hemophilia ~12,000 people have hemophilia in the U.S. 50% of male & female offspring from women who carry the gene get hemophilia 100% of female offspring from men who have hemophilia develop hemophilia About 1/3 of cases come from spontaneous gene mutation What causes hemophilia? When clotting does not form fibrin to stop the bleeding, excessive bleeding occurs Inherited sex-linked recessive trait with the defective gene on the X chromosome Females are the carrier of hemophilia Since females have two X chromosomes if one carries hemophilia the other will dominate and produce the clotting factors Males on the other hand only need one bad gene to be a hemophilic Symptoms of Hemophilia Easy bruising or bleeding Spontaneous bleeding into the joints Gastrointestinal and urinary tract bleeding Prolonged bleeding from cuts, tooth extraction or surgery Can also experience blood in stool and urine In women, it can cause excessive bleeding during menstruation joints most commonly affected by Hemophilia. It most often occurs at the knees, hips, ankles, shoulders, and elbows muscles that bleed with Hemophilia are those in the the upper arm, upper leg (front and back), the calf and the front of the groin Detection of Hemophilia When a baby starts to crawl the parents may notice bruises on stomach, chest, buttock, and back. The baby may also be fussy, not wanting to walk or crawl Other symptoms include long nosebleeds, excessive bleeding from biting down on the lips or tongue, excessive bleeding following a tooth extraction, excessive bleeding following surgery and blood in the urine. history of hemophilia In the Talmud (Jewish writings, 2nd century AD) it is stated that a male does not have to be circumcised if 2 brothers had already died from excessive bleeding Arab physician, Albucasis took notes on many males who died of excessive bleeding in the 12th century In 1803 the first case of hemophilia was recorded by Dr. John Conrad Otto who traced the disease back 3 generations to a family of males who had what he called, “a hemorrhagic disposition existing in certain families” The word hemophilia first appeared in a study of University of Zurich by Hopff in 1828. From Queen Victoria of England the gene passed to many different monarchies. Her granddaughter, Alexandra, was married to the Russian Tsar, Nicholas and gave birth to Alexei, a son born with hemophilia. That time, the monk named Rasputin performing “miracles” in the court was asked to cure the disease that inflicted Alexei. It’s been said that his hypnosis was able to slow down the bleeding of the young boy, and diminish his pain. In return, the Tsar gave him more power and authority in his court as a faith-healer. life span of people with Hemophilia Year age at the time of death Available Treatments Before 1938 11 none Before 1968 20 Plasma or Whole blood transfusions 1968 Less than 40 Cryoprecipitate 1983 64 Freeze dried clotting factors 1988 40 ( impact of aids) Freeze dried clotting factors 1999 Normal life span Factors produced by genetic engineering Modern Treatments In large injuries, the only way to stop bleeding is to replace the missing clotting factor. Clotting factor comes as a dried powder that is mixed with water. Some are plasma-derived and come right from donated human plasma. The mixture is injected as an IV and can now be done at home as well as in the hospital. If the hemophilia is severe, routine infusions of blood might be necessary which are called prophylactics. Von Willebrand disease (vWD) is the most common hereditary coagulation abnormality described in humans (also in Dogs) It arises from a qualitative or quantitative deficiency of von Willebrand factor (vWF), a multimeric protein that is required for platelet adhesion. vWF is named after Dr. Erik von Willebrand, a Finnish doctor who in 1924 first described a hereditary bleeding disorder in families from the Åland islands who had a tendency for cutaneous and mucosal bleeding, including menorrhagia. von Willebrand distinguished von Willebrand disease (vWD) from haemophilia and other forms of bleeding diathesis without identifying the cause. In the 1950s, vWD was shown to be caused by a plasma factor deficiency in the 1970s, the vWF protein was purified Von Willebrand Factor a large multimeric glycoprotein present in plasma can be >20,000 kDa with >80 subunits (250 kDa). Only the large multimers are functional vWF monomer contains 2050 amino acid. produced constitutively in endothelium (in the Weibel-Palade bodies) megakaryocytes (α-granules of platelets) subendothelial connective tissue. monomer contains specific domains with specific function the D'/D3 domain binds to Factor VIII the A1 domain binds to platelet gp1b-receptor, heparin and possibly collagen the A3 domain binds to collagen the C1 domain, binds to platelet integrin αIIbβ3 the "cysteine knot" domain (at C-terminal end), which vWF shares with PDGF, -β TGFβ) and β- human chorionic gonadotropin von Willebrand factor FUNCTION primarily binds to Factor VIII important for platelet adhesion (wound sites). vWF also binds to a number of cells and other molecules: Factor VIII is bound to vWF whilst inactive in circulation; Factor VIII degrades rapidly when not bound to vWF. Factor VIII is released from vWF by the action of thrombin. vWF binds to collagen, e.g., when it is exposed in endothelial cells due to damage occurring to the blood vessel. vWF binds to platelet gpIb when it forms a complex with gpIX and gpV; this binding occurs under all circumstances, but is most efficient under high shear stress (i.e., rapid blood flow in narrow blood vessels). vWF binds to other platelet receptors when they are activated, e.g., by thrombin (i.e., when coagulation has been stimulated). Classification of vWD There are three hereditary types of vWD Type 1 (60-80% of all vWD cases) a quantitative defect (heterozygous defective gene) may not have clearly impaired clotting most patients usually leads a nearly normal life. Bleeding may be a problem following surgery, noticeable easy bruising or menorrhagia (heavy periods). Decreased levels of vWF are detected (10-45% of normal, i.e. 10-45 IU). Classification of vWD Type 2 (20-30%) with normal levels of vWF is a qualitative defect having mild bleeding tendency. The multimers are structurally abnormal, or subgroups of large or small multimers are absent. Four subtypes: Type 2A is an abnormality of the synthesis/protelysis of the vWF multimers resulting in small multimer units. Factor VIII binding is normal. Type 2B is a "gain of function" defect leading to spotaneous binding to platelets resulting rapid clearance of platelets and large vWF multimers. A mild thrombocytopaenia may occur. The large vWF multimers are absent in the circulation and the Factor VIII binding is normal. Type 2M is caused by decreased or absent binding to GPIb on the platelets. Factor VIII binding is normal. Type 2N (Normandy) is a deficiency of the binding of vWF to factor VIII. vWF antigen level and functional test results remains normal but has a low factor VIII. This has probably lead to some 2N patients being misdiagnosed in the past as having hemophilia A. Classification of vWD Type 3 the most severe form of vWD homozygous for the defective gene may have severe mucosal bleeding reduced or absent platelet and plasma VWF (<5U/dL) may have sufficiently low factor VIII may have occasional hemarthoses (joint bleeding) Hemostasis Disorders: Bleeding Disorders Inability to synthesize procoagulants by the liver results in severe bleeding disorders Causes can range from vitamin K deficiency to hepatitis and cirrhosis Inability to absorb fat can lead to vitamin K deficiencies as it is a fat-soluble substance and is absorbed along with fat Liver disease can also prevent the liver from producing bile, which is required for fat and vitamin K absorption Prevention of Undesirable Clots Substances used to prevent undesirable clots include: Aspirin – an antiprostaglandin that inhibits thromboxane A2 Heparin – an anticoagulant used clinically for pre- and postoperative cardiac care Warfarin – used for those prone to atrial fibrillation Anticoagulant drugs for thromboembolism Drug Class Prototype Action Effect Anticoagulant Heparin Parenteral Inactivation of clotting Factors Prevent venous Thrombosis Anticoagulant Warfarin Oral Decrease synthesis of Clotting factors Prevent venous Thrombosis Antiplatelet drugs Decrease platelet Prevent arterial Thrombosis aggregation Aspirin Thrombolytic Streptokinase Fibinolysis Drugs Breakdown of thrombi Heparin and its mechanism of action Heparin antithrombin III Thrombin Sulphated carbohydrate Purified from bovine lungs Different size Active in vitro and in vivo Administration Parenteral (only IV or deep s.c.) Do not inject IM Half-life 1 - 5 hrs - monitor aPTT Adverse effect – hemorrhage Antidote - protamine sulphate Heparin antithrombin III Thrombin antiThrombin thrombin III Descarboxy Prothrombin Prothrombin Vitamin KRED Oral anticoagulants: warfarin, dicumarol Vitamin KOX NAD NADH Warfarin Isolated from clover leaves Structurally related to vitamin K Inhibits production of vit K dependent clotting factors (VII, IX, X) binds to albumin Clearance is slow - 36 hrs Overdose - reversed by vitamin K infusion Can cross placenta - prohibited during late pregnancies! Aspirin (Antiplatelet drugs) Prevents platelet aggregation /adhesion Clinical use - prevents arterial thrombus Myocardial infarction (MI), stroke, heart valve replacement and shunts Other antiplatelet drugs are - Dipyridamole, sulfinpyrazone and Ticlopidine Mechanism of action Aspirin inhibits cyclooxygenase (COX) COX is a key enzyme involved in the synthesis of thromboxane 2 (prostaglandins) Inhibits platelet aggregation Prophylactic use of Aspirin Low dose daily ( 180 mg/day) Prevents ischemic attack (ministroke) and MI 335 mg/day reduced the risk of heart attack in patients over 50 More than 1000 mg/day NO EFFECT High dose inhibits prostacyclin synthesis in cells surrounding vessels. PS normally prevents platelet aggregation. Therefore, inhibition of PS leads to abrogation of the prophylactic benefit of Aspirin Contraindication - DO NOT give to patients with glucose 6-PO4 dehydrogenase deficiency Drug interaction- prototype Warfarin Category Drugs that Promote bleeding Drugs that decrease Warfarin activity Drugs that Increase Warfarin Activity Mechanism Representative Drugs Inhibition of platelets Aspirin Inhibition of clotting Factors heparin antimetabolites Induction of Barbiturates metabolizing Enzymes Phenytoin Promote clotting factor Vitamin K Synthesis Colestipol Reduced absorption Cholestyramine Decrease binding to Albumin Aspirin, Sulfonamides Inhibit Degradation Cimetidine, Disulfiram Decrease synthesis of Antibiotics (oral) Tests of Hemostasis Screening tests Bleeding.T - To test Platelet & BV function Prothrombin.T – Extrinsic aPTT – Instrinsic Thrombin.T – Both paths (DIC) Specific tests Factor assays Tests of thrombosis Platelet function studies: Adhesion, Aggregation, Release & PG pathway tests. Bone Marrow study Prothrombin time (PT) Tissue Thromboplastin factor III Mix with phospholipid extract Add calcium and blood sample Determine clotting time Generally 12 - 14 seconds Used to detect defects in extrinsic pathway Activated partial thromboplastin time (APTT) Blood sample + EDTA or Citrate No clot (recalcification will result in clot in ~2 - 4 min) Add calcium Mix with negatively charged phospholipid Kaoline (aluminum silicate) Determine clotting time Generally clotting occurs in 26 to 33 seconds Used to detect defects in the intrinsic pathway Diagnosis of coagulation defects Prolonged APTT No change in PT Defective Intrinsic Pathway No change in APTT Prolonged PT Defective Extrinsic Pathway Prolonged APTT Prolonged PT Defective in Common pathway