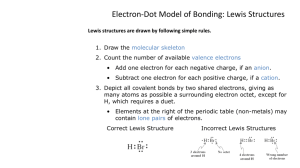
Lewis Structures
advertisement

Writing Lewis Structures for Molecular (Covalent) Compounds 1. 2. 3. 4. 5. 6. sketch arrangement of atoms (determine central atom) determine number of valence eplace two e- in each bond complete octets on non-central atoms place any remaining e- on central atom IF central atom has less than an octet, form multiple bonds Drawing Lewis Structures Draw the Lewis structure for the NO21- ion. First, determine the atomic arrangement. O N O Next, determine the total number of valence electrons. 5 electrons from the N 6 electrons from each O for 12 more electrons 1 additional electron because it is a monoanion 18 total valence electrons Use the electrons to make electron-pair bonds between the central and outer atoms. O N O 18 - 4 = 14 electrons remain Complete the octet of each outer atom. .. N .. O .. .. .. O .. 14 - 12 = 2 electrons remaining If there are any remaining electrons, put them on the central atom. .. .. N .. O .. .. .. O .. All of the valence electrons have now been used. If, and only if, the central atom has fewer than eight electrons, complete its octet by using nonbonding electron pairs from outer atoms to make multiple bonds. .. .. N .. O .. .. .. O .. In the final Lewis structure, each atom should have a complete octet of electrons. If it is an ion, indicate the charge outside of brackets. .. .. N .. O .. .. O .. 1-