polymer
advertisement

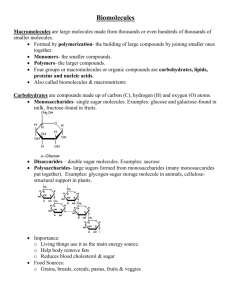
3-2: Water and Solutions What makes water a unique substance? How does the presence of substances dissolved in water affect the properties of water? Intro to Water The bodies of all living organisms; humans to trees to jellyfish, are composed of cells that are mostly water The chemical reactions of life take place in an aqueous environment (cytoplasm, water, humid air) Water has very unique properties Describe the structure of a water molecule. H2O 2 Hydrogen atoms + 1 Oxygen atom Polar covalent bonding Electrons are closer to oxygen Bent/ angled, not straight line Because of polarity – waters are attracted to other water molecules Structure of water molecules Explain how water’s polar nature affects its ability to dissolve substances Water is a polar compound The Oxygen region is slightly negative and the Hydrogen regions are slightly positive, but the overall compound is neutral. Water dissolves other polar compounds (protein and sugar) “like dissolves like” Water also dissolves ionic compounds (NaCl) because the salt ionizes/dissociates and it attracted to the polar regions. (see pg. 53) ION concentration is CRUCIAL to life functions. Hydrogen Bonding The negative regions near the Oxygen of one water attract the positive regions near the Hydrogen of another water. Waters link to other waters with ‘hydrogen bonds’ which are very weak There are so many H bonds that collectively they are strong List two of water’s properties that result from hydrogen bonding 1. 2. Cohesion and adhesion = water bonding to other waters and water being attracted to other substances like glass. Temperature moderation = water has to gain lots of heat to increase in temperature and must give up a lot of heat to decrease in temperature Impact of waters unique properties Body temp stays steady Evaporative cooling Coastal temperatures are buffered Lakes don’t freeze solid Plants are able to PULL water up stem instead of relying on push of air pressure 3-3: Carbon Compounds What are chemicals of life made from? What is the role of carbohydrates in cells? What do lipids do? What determines the functions of a protein? What do nucleic acids do? Building Blocks of Life Organic compounds = those containing chains or rings of carbon covalently bonded together. C typically bonds to H, O, N, P, S Large carbons molecules are called macromolecules Polymerization Smaller, simpler molecules used to build large compounds are called monomers Chains of repeating small units are called polymers Monomers are linked using a condensation reaction Polymers are split using hydrolysis Analogies for Polymerization Monomer (small units) Initial joining – polymerization Final product polymer Letter, letters of alphabet Words paragraph Student = 1 Elementary class = 25 Graduating class = 250 Amino acids Polypeptide chain Protein Fatty acids and glycerol Lipids Nitrogenous bases (A, T, C, G, U) Nucleotides; genes DNA and RNA Saccharides, monosaccharides, simple sugars Disaccharides Polysaccharides; complex carbs, starch, cellulose, etc ENERGY It requires a lot of energy to run a cell The currency of the cell is called ATP Adenosine tri phosphate The three phosphate groups (PO4-3) have much bond energy ATP + H2O ADP + P + energy ATP – Adenosine tri phosphate Three (tri) phosphate groups Adenosine Carbohydrates Monosaccharides Disaccharides Simple sugars Blood glucose Immediate energy Made in photosynthesis Two sugars Sucrose or table sugar Transport format in plants Polysaccharides ‘complex carbohydrates Many hundreds of sugars “starch” Storage form of sugar and energy Starch, glycogen and cellulose C A R B O H Y D R A T E S Functions Monomer Version Saccharide glucose Monosaccharides Energy; Immediate Glucose, fructose energy FUEL Sources Blood sugar – REQUIRED by ALL cells Corn syrup, fruit sugars Polymers Starch, carbs. Disaccharides sucrose Transport of energy in plants Refined sugar aka “table sugar” Fruits vegetables Elements Polysaccharides Starch, ‘complex carbs’, fiber, wood Energy storage; “stored energy” or “long term energy” Grains – corn, wheat, rice, and things made with flour, oats Potatoes, barley CH2O Carbohydrates are molecules made of sugars. A sugar contains carbon, hydrogen, and oxygen in a ratio of 1:2:1. Glucose is a common sugar found in grape juice. Lipids Very large Nonpolar ( not water soluble ) Glycerol + fatty acids Hydrophilic and hydrophobic regions are important to construction of cell membranes ( phospholipids) In the body fat has many forms and functions that include Insulation, both thermal and electrical Hormonal messengers Cushion of vital organs Energy STORAGE – for long, long term storage – “emergency” L I P I D S Monomer Versions Functions Sources Glycerol Fatty acid Fats Saturated Unsaturated Insulation •Thermal •Electrical Meats/steak fat Waxes Cushion Polymer Lipids Elements CH (few O) “fuels” “Hydrocarbons” Whole milk Oils Seeds/nuts Cell membrane Olives Phospholipids Hormones Egg-yolk STORED energy Oil – fried foods Proteins Very important Made of pieces called amino acids whose sequence is coded for by DNA Can be used for the following: Muscle contraction Neurotransmitters Transporters like hemoglobin Defenses like antigens and antibodies Structures like nails, hair, tendon and hooves Enzymes for reactions Messengers like insulin Channeling of compounds into cells P R O T E I N S Elements Versions Functions Sources C H O and N, some S Many forms Can be globular, sheet like see notes on previous slide Meat/tissue Monomers Specific Amino acids shape Milk Dairy products Seeds/ nuts/ beans Soy Peanut butter Polymers Egg (white) Polypeptides or proteins Nucleic acids These molecules are for storage of INFORMATION The DNA and RNA code for how to make the proteins that compose the cell and run the cell Monomers are nucleotides Adenine Guanine Cytosine Thymine and Uracil N U C L E I C A C I D ELEMENTS CHNOS and P Monomers Nitrogenous bases and Nucleotides Polymers DNA RNA Versions Functions DNA RNA Store information Sources Anything with cells – eat cell Regulate the – eat nucleus building of proteins – get a supply of nucleotides Cell division and growth RNA makes up ribosomes “YOU ARE WHAT YOU EAT” 3-4: Energy and Metabolism Where so living things get energy? How do chemical reactions occur? Why are enzymes important to living organisms? Changing Matter A key feature of life is change, and change requires energy Energy can be in many forms, including of heat, light or chemical energy. Physical change form or shape of matter changes (sheet of paper to shredded document) Chemical change substances change (piece of tree burns and becomes CO2 , H2O vapor and ash) Conservation Conservation of Mass Matter is neither created nor destroyed in any change. The same mass (amount of matter) is there before and after the reaction Conservation of Energy Every change in matter requires a change in energy. Energy may change forms, but the total amount of energy does not change. Chemical Reactions During a chemical change, a chemical reaction takes place where bonds between atoms are broken and new bonds form. reactant(s) CO2 + H2O product(s) H2CO3 “carbon dioxide plus water yield carboxylic acid” 2 Important Reactions Photosynthesis 6 CO2 + 6 H2O C6H12O6 + 6 O2 Cellular Respiration C6H12O6 + 6 O2 6 CO2 + 6 H2O sunlight and chlorophyll Activation Energy Reactions need the right conditions Atoms must be close enough, long enough for new bonds to form Collisions must have enough energy Molecules must have the correct alignment Energy to ‘start’ a reaction is called Activation Energy Biological Reactions Living organisms have multiple chemical reactions occurring constantly These are directed by enzymes Enzymes molecules that increase the speed of a biochemical reaction without being consumed (part of) the reaction. Enzymes are made of protein. They could also be called catalysts Enzymes Substrate – what the enzyme attaches to (the reactant) Active site – where the enzyme and the substrate fit together Substrate(s) + enzyme product(s) + enzyme S + E SE complex P + E