Periodic Table
advertisement
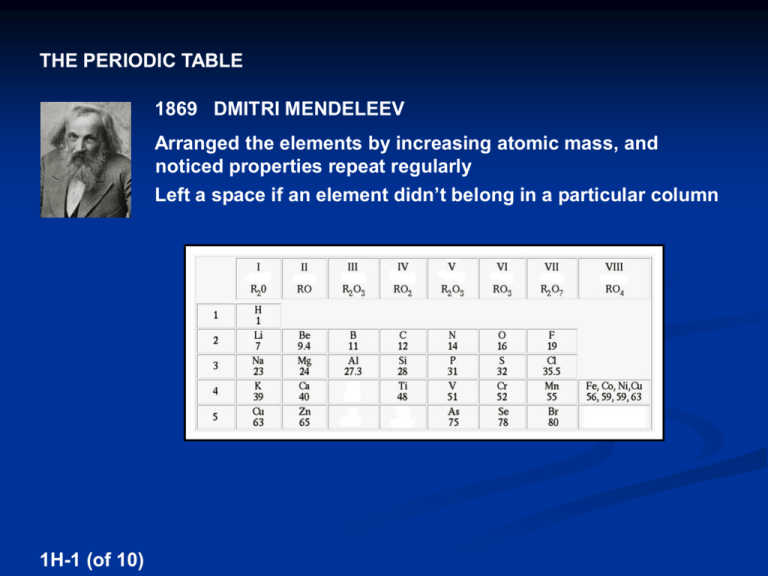
THE PERIODIC TABLE 1869 DMITRI MENDELEEV Arranged the elements by increasing atomic mass, and noticed properties repeat regularly Left a space if an element didn’t belong in a particular column 1H-1 (of 10) Mendeleev stated the PERIODIC LAW: The properties of the chemical elements are not arbitrary, but vary with their atomic masses in a systematic way Mendeleev was able to predict properties of undiscovered elements However, accurate atomic mass determinations showed errors in the table 1H-2 (of 10) 1912 HENRY MOSELEY Measured the frequency of x-rays from excited atoms Found they were proportional to the ATOMIC NUMBER of the atoms, not the atomic mass 1H-3 (of 10) 1912 HENRY MOSELEY This work changed the PERIODIC LAW: The properties of the chemical elements vary with atomic number 1H-4 (of 10) PERIOD or SERIES – A row GROUP or FAMILY – A column Elements in columns have similar properties because they have the same number of valence electrons 1H-5 (of 10) PERIODIC TRENDS OF ATOMS The attraction of either (1) outer shell electrons or (2) free electrons to the nucleus of an atom depends on (1) The number of protons in nucleus (2) The number of energy levels blocking or SHIELDING the nucleus from the outer shell or free electrons 1H-6 (of 10) 1) ATOMIC RADIUS – The distance from the nucleus to the outer shell of an atom 1H-7 (of 10) PERIODIC TRENDS IN ATOMIC RADII Period – The atomic radii decrease moving to the right the outer shell electrons are attracted by an increasing nuclear charge, while the shielding of the nuclear charge remains the same Group – The atomic radii increase moving down a column even though the nuclear charge is increasing, the outer shell electrons are more shielded from the nuclear charge Atom with the largest atomic radius ? Atom with the smallest atomic radius? 1H-8 (of 10) 1H-9 (of 10) EXCEPTIONS TO THE TRENDS IN ATOMIC RADII Group Atoms in the 5d sublevel section of the periodic table are not larger than atoms in the 4d sublevel section Radii V Increasing Factor Decreasing Factor (Z = 23) 0.122 nm Nb (Z = 41) 0.134 nm 1 more EL 18 more p+ Ta (Z = 73) 0.134 nm 1 more EL 32 more p+ This is called the LANTHANIDE CONTRACTION 1I-10 (of 10) 2) IONIZATION ENERGY (IE) – The energy change for the removal of an electron from a gaseous atom X (g) → X+ (g) + eEndothermic – A process in which energy is absorbed (∆E is positive) Exothermic – A process in which energy is released (∆E is negative) Ionization Energies are always endothermic 1I-1 (of 15) SUCCESSIVE IONIZATION ENERGIES → Mg+ (g) + e- 1st IE = 736 kJ/mol Mg+ (g) → Mg2+ (g) + e- 2nd IE = 1,451 kJ/mol Mg2+ (g) → Mg3+ (g) + e- 3rd IE = 7,728 kJ/mol Mg3+ (g) → Mg4+ (g) + e- 4th IE = 10,534 kJ/mol Mg (g) Successive ionization energies always increase because successive ions have less e--e- repulsion 1I-2 (of 15) SUCCESSIVE IONIZATION ENERGIES → Mg+ (g) + e- 1st IE = 736 kJ/mol Mg+ (g) → Mg2+ (g) + e- 2nd IE = 1,451 kJ/mol Mg2+ (g) → Mg3+ (g) + e- 3rd IE = 7,728 kJ/mol Mg3+ (g) → Mg4+ (g) + e- 4th IE = 10,534 kJ/mol Mg (g) Successive ionization energies always increase because successive ions have less e--e- repulsion Small ionization energies occur when removed e-s are valence e-s, which are the most shielded from the nuclear charge 1I-3 (of 15) Atom 1 Atom 2 1st IE = 496 kJ/mol 1st IE = 578 kJ/mol 2nd IE = 4,562 kJ/mol 2nd IE = 1,817 kJ/mol 3rd IE = 6,912 kJ/mol 3rd IE = 2,745 kJ/mol 4th IE = 9,543 kJ/mol 4th IE = 11,577 kJ/mol Determine which atom is sodium and which atom is aluminum Na 1I-4 (of 15) Al PERIODIC TRENDS IN 1st IONIZATION ENERGIES Period – The 1st IE increases moving to the right the removed electron is attracted by an increasing nuclear charge, while the shielding of the nuclear charge remains the same Group – The 1st IE decreases moving down a column even though the nuclear charge is increasing, the removed electron is more shielded from the nuclear charge Atom with the highest 1st IE ? 1I-5 (of 15) Atom with the lowest 1st IE? 1I-6 (of 15) EXCEPTIONS TO THE TRENDS IN 1st IONIZATION ENERGIES Period Li Be 519 900 1s B ↑↓ ___ B C N O F Ne 799 1088 1406 1314 1682 2080 2s ↑↓ ___ 2p ↑___ ___ ___ The removed e- for B is in a p orbital, whereas the removed e- for Be is in an s orbital - e-s in p orbitals are more shielded from the nuclear charge than e-s in s orbitals 1I-7 (of 15) EXCEPTIONS TO THE TRENDS IN 1st IONIZATION ENERGIES Period Li Be 519 900 1s O ↑↓ ___ B C N O F Ne 799 1088 1406 1314 1682 2080 2s 2p ↑↓ ↑↓ ↑ ↑ ___ ___ ___ ___ The removed e- for O is paired and experiencing e--e- repulsion, whereas the removed e- for N is not 1I-8 (of 15) 3) ELECTRON AFFINITY (EA) – The energy change for the addition of an electron to a gaseous atom X (g) + e- → X- (g) Electron Affinities are always exothermic 1I-9 (of 15) PERIODIC TRENDS IN ELECTRON AFFINITIES Period – EA increases (becomes more exothermic) moving to the right a free electron is attracted by an increasing nuclear charge, while the shielding of the nuclear charge remains the same (except noble gases) Group – EA decreases (becomes less exothermic) moving down a column even though the nuclear charge is increasing, a free electron is more shielded from the nuclear charge 1I-10 (of 15) 1I-11 (of 15) EXCEPTIONS TO THE TRENDS IN 1st ELECTRON AFFINITIES Group In the p block of the periodic table, adding e-s to small atoms (those in the 2nd period) results in large e--e- repulsion, so their 1st EA’s are slightly less exothermic than atoms in the 3rd period F -328 kJ/mol Cl -349 kJ/mol Br -343 kJ/mol I -295 kJ/mol 1I-12 (of 15) ← element with the highest EA EXCEPTIONS TO THE TRENDS IN 1st ELECTRON AFFINITIES Period K Ca Ga Ge As Se Br Kr -49 ~0 -76 -116 -75 -195 -325 ~0 4s ↑↓ 3d 4p Ca [Ar] ___ ___ ___ ___ ___ ___ ___ ___ ___ Kr [Ar] ___ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ___ ___ ___ ___ ___ ___ ___ ___ ↑↓ ↑↓ ↑↓ The added e- will go into an orbital of a new sublevel, where it will be very shielded from the nuclear charge 1I-13 (of 15) EXCEPTIONS TO THE TRENDS IN 1st ELECTRON AFFINITIES Period As K Ca Ga Ge As Se Br Kr -49 ~0 -76 -116 -75 -195 -325 ~0 [Ar] 4s 3d 4p ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ___ ___ ___ ___ ___ ___ ___ ___ ___ ↑ The added e- will experience e--e- repulsion 1I-14 (of 15) ↑ ↑ EXCEPTIONS TO THE TRENDS IN 1st ELECTRON AFFINITIES Period K Ca Ga Ge As Se Br Kr -49 ~0 -76 -116 -75 -195 -325 ~0 What other two elements in the 4th period would you expect to have low electron affinities? 1I-15 (of 15) Bismuth (Z = 83) – The element of highest atomic number with at least one stable isotope Plutonium (Z = 94) – The element of highest atomic number found naturally on Earth 1J-13 (of 13) METALS Physical Properties – Low number of valence electrons, metallic luster, malleable, ductile, conductors of heat and electricity Solids are brilliant white (or silver) except copper (red) and gold (yellow), mercury is a liquid Chemical Properties – Due to the low ionization energies of their valence electrons, they can lose their valence electrons to produce positive ions (called CATIONS) 1J-1 (of 13) Group 1 Group 2 - Alkali Metals - Alkaline Earth Metals Group 11 8, 9, 10 8, 9, 10 d Block - f Block - Inner Transition Metals (Lanthanides and Actinides) 1J-2 (of 13) Coinage Metals Fe-Co-Ni Triad - The Ferromagnetic Metals Noble Metals (Ru, Rh, Pd, Os, Ir, Pt) Transition Metals Metal Hardness Group 1 metals are soft, hardness increases to Group 6, and then hardness decreases to Group 16 1J-3 (of 13) Metal Activity Group 1 metals are extremely active, and activity decreases to Group 11, with the Noble Metals, Coinage Metals, and Hg being very inactive Al and Zn are very active, and activity decreases down and to the right 1J-4 (of 13) NONMETALS Physical Properties – High number of valence electrons, opposite of metals Some are crystalline solids, bromine is a liquid, and some are gases Chemical Properties – Due to their highly exothermic electron affinities, they can gain electrons until their outer shells are full to produce negative ions (called ANIONS) 1J-5 (of 13) Group 18 Group 17 - Noble Gases - Halogens Hydrogen - A group of its own in that it can form 1+ and 1- ions 1J-6 (of 13) Hydrogen - Colorless gas (H2) Helium - Colorless gas (He) 1J-7 (of 13) Boron - Crystalline brown Carbon - Crystalline as diamond, graphite, or buckminsterfullerene; amorphous as charcoal ALLOTROPES – Forms of an element with different interatomic bonding, so different properties 1J-8 (of 13) Nitrogen - Colorless gas (N2) Oxygen - Colorless gas allotropes dioxygen (O2) and ozone (O3) Phosphorus - Crystalline red, white (P4), and black allotropes Sulfur - Crystalline yellow (S8 rings) and other allotropes (S8 chains) 1J-9 (of 13) Fluorine - Pale yellow-green gas (F2) Chlorine - Yellow-green gas (Cl2) Bromine - Orange liquid (Br2) Iodine - Crystalline black (I2), sublimes to violet vapor Noble Gases - Colorless monatomic gases 1J-10 (of 13) Nonmetal Activity Group 17 nonmetals are extremely active, and activity decreases down and to the left H is active, and the Noble Gases are inert 1J-11 (of 13) METALLOIDS Intermediate number of valence electrons, properties of metals and nonmetals 1J-12 (of 13)









