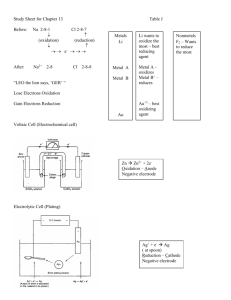
Redox
advertisement

Good Morning Ladies! 1. Questions before the test? 2. TEST In both fission and fusion reactions, energy is… RELEASED because… E = mc2 Energy = mass x speed of light squared Mass is converted to energy 100 g samples of both radium-226 and radon-222 were left in a chemical laboratory in 1950. Which sample poses the least risk today? Half-life of radium-226 is 1600y Half-life of radon-222 is 3.82d • radon-222 because it has a shorter half-life • radon-222 because it decays faster Good Afternoon Ladies! 1. Questions before the test? 2. TEST ……………. 3. Begin Oxidation-Reduction Reactions Unit In both fission and fusion reactions, energy is… RELEASED because… E = mc2 Energy = mass x speed of light squared Mass is converted to energy 100 g samples of both radium-226 and radon-222 were left in a chemical laboratory in 1950. Which sample poses the least risk today? Half-life of radium-226 is 1600y Half-life of radon-222 is 3.82d • radon-222 because it has a shorter half-life • radon-222 because it decays faster Good Morning Ladies! • Prayer • Regents Review Resources • Begin Oxidation-Reduction Reactions Unit – What are oxidation-reduction reactions? – Assigning oxidation numbers HW: Redox 1 DUE MONDAY Also: Simulating Half-Life Lab DUE next Friday Oxidation-Reduction Reactions *What kind of chemical reaction is responsible for burning, rusting, bleaching clothes, powering your cell phone as well as your body, and giving fireflies their glow? • Oxidation-Reduction Reactions, also called REDOX reactions I. What are Redox Reactions? • Redox reactions involve the transfer of ELECTRONS (e–) between different atoms Na + Cl → Na + Cl Oxidation Reduction * CONSERVATION OF CHARGE: oxidation and reduction happen simultaneously (at the same time) – you can’t have one without the other Remember: LEO the lion says GER (or LEO the liger says GER) • LOSING ELECTRONS is OXIDATION • GAINING ELECTRONS is REDUCTION Good Afternoon Ladies! • Determining if a reaction is redox • Assigning oxidation numbers HW: Redox 1 DUE MONDAY Also: Simulating Half-Life Lab DUE next Friday II. Determining if a reaction is a redox reaction • If the oxidation number/state of an atom changes from reactant to product, a redox reaction has occurred *Note: Double replacement reactions are NOT redox reactions A. Assigning Oxidation Numbers (listed on the Periodic Table) 1. If a species is all by itself (not combined with a different element), its oxidation number is ZERO Ex: Mg O2 S2 Cu H2 A. Assigning Oxidation Numbers (listed on the Periodic Table) 2. In a polyatomic ion, the elements’ oxidation numbers add up to equal the CHARGE on the ion * SOS: you must multiply the Subscript by the Oxidation number to get the Sum NH4 + – ClO A. Assigning Oxidation Numbers (listed on the Periodic Table) 2. In a polyatomic ion, the elements’ oxidation numbers add up to equal the CHARGE on the ion * SOS: you must multiply the Subscript by the Oxidation number to get the Sum SO3 2– SO4 2– A. Assigning Oxidation Numbers (listed on the Periodic Table) 3. In a compound, the oxidation numbers of the elements add up to ZERO (compounds are neutral) * SOS: you must multiply the Subscript by the Oxidation number to get the Sum NaCl A. Assigning Oxidation Numbers (listed on the Periodic Table) 3. In a compound, the oxidation numbers of the elements add up to ZERO (compounds are neutral) • SOS: you must multiply the Subscript by the Oxidation number to get the Sum NH4Cl A. Assigning Oxidation Numbers (listed on the Periodic Table) 3. In a compound, the oxidation numbers of the elements add up to ZERO (compounds are neutral) • SOS: you must multiply the Subscript by the Oxidation number to get the Sum H2SO3 More Examples a.) What is the oxidation number of chlorine in iron (III) chloride? More Examples b.) What is the oxidation state of chromium in potassium chromate? B. Checking for Changes in Oxidation Numbers 2 Na (s) + Cl2 (g) → 2NaCl (s) • • • • The oxidation # on Cl Nachanges changesfrom from00to to–1 +1 Nagains loseselectrons electrons Cl Na oxidized Cl isisreduced Naisisthe theoxidizing reducingagent agent Cl B. Checking for Changes in Oxidation Numbers 2Mg (s) • • • • + O2 (g) → 2MgO (s) Mgchanges changesfrom from0 0toto–2+2 The oxidation # on O Mggains loseselectrons electrons O Mgisisreduced oxidized O Mgisisthe theoxidizing reducingagent agent O Orange Packet • #s 1, 21, 23, 25 Good Morning Ladies! • • • • Prayer Review Videos on Wikispace! Trade & Grade Redox 1 (collect) Writing Oxidation & Reduction Half-Reactions HW: Redox 2 DUE WEDNESDAY Reminder: Simulating Half-Life Lab DUE FRIDAY Good Afternoon Ladies! • Review Videos on Wikispace! • Trade & Grade Redox 1 (collect) • Writing Oxidation & Reduction Half-Reactions HW: Redox 2 DUE WEDNESDAY Reminder: Simulating Half-Life Lab DUE FRIDAY a.) Zn + 2HCl → ZnCl2 + H2 b.) 4Al + 3O2 → 2Al2O3 c. For soldiers on the front lines or on special remote missions, a hot meal can make a big difference. Instead of building a fire or carrying a stove with them, soldiers simply break open a bag and mix some magnesium metal with water to heat their food. This reaction is an example of a redox reaction, producing magnesium hydroxide, hydrogen gas, and heat as products. In the space below, write the oxidation and reduction half-reactions. Whiteboard Practice Write the oxidation and reduction half-reactions for the following redox reactions Zn + HNO3 Zn(NO3)2 + NO2 + H2O Sn + HNO3 + H2O H2SnO3 + NO NaClO + H2S NaCl + H2SO4 Orange Packet #s 2, 3, 19, 20, 22, 24, 26-30, 32-35 Good Morning Ladies! (TUES) • • • • • Prayer Gummy Bear Sacrifice Breathalyzer Demo Balancing Redox Reactions Pass back Nuclear Chemistry Test HW: Redox 2 DUE TOMORROW Reminder: Simulating Half-Life Lab DUE FRIDAY In the presence of oxygen, iron forms iron (III) oxide, more commonly known as rust. In the space below, write the balanced equation for the chemical process of the corrosion of iron using the oxidation and reduction half-reaction method. What causes fruit to turn brown? • Oxidation of compounds in fruit after exposure to oxygen in the air causes a change of color (browning) of fruit Oxidation (losing electrons) is a process that can be very damaging to living things. • results in the creation of free radicals, which are chemical species that have unpaired electrons (they are unhappy – they don’t have a completed valence shell) • free radicals will rip electrons away from other compounds in living things, such as proteins or molecules of DNA, causing damage which can lead to cancer • Antioxidants get rid of free radicals – Vitamins A, C, and E are antioxidants Good Morning Ladies! (WED) Please place your Redox 2 HW in the bin • Prayer • Two Types of Electrochemical Cells – Voltaic cells – Electrolytic cells Reminder: Simulating Half-Life Lab DUE FRIDAY Heads up HW: Redox 3 DUE TUESDAY *Redox Unit TEST next WEDNESDAY Good Afternoon Ladies! (WED) Please place your Redox 2 HW in the bin • Two Types of Electrochemical Cells – Voltaic cells – Electrolytic cells Reminder: Simulating Half-Life Lab DUE FRIDAY Heads up HW: Redox 3 DUE TUESDAY *Redox Unit TEST next WEDNESDAY V. Electrochemical Cells A. Use REDOX reactions to convert chemical energy into electrical energy OR convert electrical energy into chemical energy 1. Voltaic (Galvanic) Cells = batteries a.) SPONTANEOUS redox reactions – NO energy put in (energy is released) Use Table J to determine what is oxidized and what is reduced Higher on Table J is OXIDIZED Lower on Table J is REDUCED *Parts to Know* Electrodes: the places where oxidation or reduction happens o Anode: the site of oxidation (An Ox) o Cathode: the site of reduction (Red Cat) Wire: electrons flow through the wire from the anode (–) to the cathode (+) Salt bridge: allows IONS to flow between the electrodes to keep the charges balanced The electrodes must be separated in order to produce an electric current (flow of electrons). The energy present in the flowing electrons (ELECTRICITY) is captured and used to power other processes. b.) Diagram of a voltaic cell using Zn and Zn(NO3)2 with Cu and Cu(NO3)2 c.) Voltaic cell problems: 1. Look on Table J and find which element is higher – this element is OXIDIZED (On Table J – electrons flow DOWNHILL, spontaneously) 2. Under each beaker write “oxidation” or “reduction” 3. Label the oxidation electrode ANODE (An Ox) 4. Label the reduction electrode CATHODE (Red Cat) 5. Place a (–) charge on the anode 6. Place a (+) charge on the cathode 7. Draw in the direction of electron flow (from ANODE (–) to CATHODE (+)) 8. Write the half reactions under the correct beakers 9. Be able to describe what is occurring at the anode and cathode 10. The salt bridge allows for ions to flow between the electrodes Positive (+) ions flow toward the CATHODE (to balance the negative electrons) Negative (–) ions flow toward the ANODE (to replace the electrons that are leaving) How many of you have had cavities filled? …if you do, you have the potential to make a tiny battery IN YOUR MOUTH! • Cavities are filled with a mixture of metals including zinc (Zn), tin (Sn), copper (Cu), and silver (Ag) • If you bite down on a piece of aluminum foil, the saliva in your mouth, the aluminum foil, and the filling make a little voltaic cell that produces a tiny current that travels through your tooth to the nerve below the filling…it’s a bit UNPLEASANT! Voltaic cell animation • http://www.blackgold.ab.ca/ICT/Division4/Sci ence/Div.%204/Voltaic%20Cells/demo.htm Orange Packet #s 4-12, *16, 36, 37, 39 2. Electrolytic Cells a.) NONSPONTANEOUS redox reactions – need to put in energy b.) The species more likely to lose electrons is forced to gain electrons (On Table J – electrons are moving UPHILL, nonspontaneously) 2. Electrolytic Cells c.) Electrons still flow from the anode to the cathode, but now the signs are reversed • The anode is (+) and the cathode is (–) (electrons are being forced to travel to the negative electrode) 2. Electrolytic Cells d.) Used for 1. Electrolysis: using electricity to break apart (lyse) compounds into their elements Ex: Obtaining active elements from compounds 2NaCl (l) 2. Electrolytic Cells d.) Used for 1. Electrolysis: using electricity to break apart (lyse) compounds into their elements Ex: Obtaining active elements from compounds 2H2O (l) 2. Electrolytic Cells d.) Used for 2. Electrolytic cells can also be used for electroplating – putting a metal coating on something Anode: Cathode: Comparing & Contrasting Voltaic and Electrolytic Cells Voltaic Cells (Batteries) • SPONTANEOUS redox rxn • energy is produced (electricity) • anode is (-) • cathode is (+) Electrolytic Cells) • nonspontaneous redox rxn • energy is needed (needs electricity) • anode is (+) • cathode is (-) Comparing & Contrasting Voltaic and Electrolytic Cells Voltaic Cells (Batteries) Electrolytic Cells) • redox reactions • anode is where oxidation happens (An Ox) • anode loses mass • cathode is where reduction happens (Red Cat) • cathode gains mass • electrons flow through wire from anode to cathode Orange Packet #s 13-15, *17, *18, 38, 40-61 Bioluminescence vs. Fluorescence • Redox reaction produces excited electrons • External energy source excites electrons • Spectral lines • UV light demos • CuSO4 in Zn • Hydrolysis of water