Maleic Anhydride Plant Process Design Report
advertisement

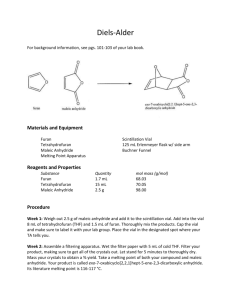
PROCESS DESIGN OF MALEIC ANHYDRIDE PLANT BY WORIL TURNER DUDLEY VIJAYA KRISHNA BODLA TABLE OF CONTENTS 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Introduction The five processes selected Product and Process Selected for Design Screening of Process Alternatives Material Balance Energy Balance Equipment Sizing Equipment Costing Heat Intergration Economic evaluation Environmental Analysis Conclusion INTRODUCTION The process design project involves designing a process plant for producing a particular product. A list of five products are selected, then from that list one product is selected for the design project. Selection of the best process for the design from a list of alternatives is then done. Material and energy balances are done, equipment sizing and costing, and then an economic evaluation of the process. Different tools of process optimization were considered for cost savings. Heat integration is done for the process to calculate the additional heating or cooling required. An environmental analysis was also done to determine the environmental impact of effluent discharge streams. Product Names Raw Materials Process References Maleic Anhydride n-butane and air Huntsmann fixed bed maleic anhydride process, Kirk Othmer Encyclopaedia of Chemical Technology by Timothy R. Felthouse, Joseph C. Burnett, Ben Horrell, Micheal J. Mummey and Yeong-Jen Kuo Citric Acid Sucrose or dextrose Fermentation of sugars to produce citric acid, Shreves Chemical Process Industries 5th Edition by George T. Austin, page 598 Acetaldehyde Oxygen, water and ethylene Oxidation of ethylene to produce acetaldehyde, Shreves Chemical Process Industries Ammonia Nitrogen and Hydrogen Ammonia process, Shreves Chemical Process Industries 5th Edition by George T. Austin page 306 Cinnamic Aldehyde Water and aldol Cinnamic aldehyde production by aldol condensation, Shreves Chemical Process Industries 5th Edition by George T. Austin page 494 PRODUCT SELECTED The unique nature of maleic anhydride's chemical structure results in a highly reactive and versatile raw material. Its unsaturated double bond and acid anhydride group lend themselves to a variety of chemical reactions. Maleic anhydride's largest use today is in the production of unsaturated polyester resins. Another significant use is in the manufacture of alkyd resins, which are in turn used in paints and coatings. Other applications where maleic anhydride is used include the production of agricultural chemicals, maleic acid, copolymers, fumaric acid, lubricant additives, surfactants and plasticizers. Future applications are anticipated to be numerous given the versatility and usefulness of the product. REACTIONS INVOLVED C4H10 + 3.5 O2 C4H2O3 + 4 H2O ∆H = -1236 kJ/mol (-295.4 kcal/mol) C4H10 + 6.5 O2 4 CO2 + 5 H2O ∆H = -2656 kJ/mol (-634.8 kcal/mol) C4H10 + 4.5 O2 4 CO + 5 H2O ∆H = -1521 kJ/mol (-363.5 kcal/mol) SCREENING OF PROCESS ALTERNATIVES There are two predominant raw materials for producing maleic anhydride, nbutane and benzene. Benzene however is a major environmental concern, because it is deemed as carcinogenic, so on environmental grounds, without even looking at raw material costs, benzene is rejected as the raw material for the maleic anhydride manufacture. The process is a high temperature process so all the components leaving the reactor are gases, so several separation options exist. The gases can be flashed, to recover water and maleic anhydride as liquids, while the other gases will remain in the vapor phase. We then consider separating water from maleic anhydride by exploiting the differences between their physical properties. A solvent can be used for the product recovery, by contacting the product gases with a liquid solvent and then separating the maleic anhydride from the solvent. A number of alternatives exist for the solvent. The conversion of butane is 85%, so recycling the unreacted butane is an option. It is decided to use a process in which a solvent is used for absorbing the maleic anhydride produced. Process Flow sheet Purge (μ92) R1 9. Splitter μ41 μ42 CM2 4. Condenser C1 µ31 µ01 µ1 1. Mixer µ32 2. Reactor μ61 µ51 3. Absorber 5. Mixer 2 6. Distillation μp C3 µ2 R1 μ62 µ81 μ71 7. Distillation Final Column CM1 8. Mixer 3 HE1 So HE2 C2 μ72 R2 μS R3 MASS BALANCE FLOW CHART Purge (μ92) R1 9. Splitter μ41 μ42 4. Condenser µ31 µ01 µ1 µ2 µ32 µ51 μ61 1. Mixer 2. Reactor 3. Absorber 5. Mixer 2 6. Distillation μp μ62 μ71 µ81 7. Distillation 8. Mixer 3 So μ72 μS Final Column MASS BALANCE Assume an inlet flow of 100 Kmol/h of butane Assume compressed air is fed in a ratio, where the amount of Oxygen is 1.5 times the amount required Using the yield of Maleic Anhydride, percentage conversion of butane and the reaction stoichiometry of the reaction material balance relations are written for the reactor For the side reactions, it is assumed that equal amounts of butane reacts to form Carbon Dioxide as for Carbon Monoxide Split factors are then specified for all the separation equipment as well as for the purge The absorber is specified to be an isothermal absorber It is assumed that the solvent entering the column doesnot contain any Maleic Anhydride For the absorber mass balance model, the Kremser equation is used to determine the number of stages, using the split factor for the key component recovery. The remaining split factors for the other components are calculated from the Kremser relationship To solve the mass balance model, the flow sheet is partitioned into two modules and the recycle broken by tearing the inlet stream to the reactor. Once the component flows to the reactor are calculated, from the mass balance model, we can sequentially calculate all the other flowrates With the flowrates calculated, distillation column temperatures can be calculated. Temperature for the vapor leaving top of the column is found from a dew point calculation. The temperature in the condenser and reboiler is calculated from a bubble point calculation It is assumed that the distillation column operates at one atmosphere of pressure. Component μ01 μ1 μ2 μ31 μ32 μ41 μ42 μ51 μ61 μ62 μ71 μ72 Maleic Anhydride 0.0 0.1 57.8 0.3 57.5 0.1 0.2 57.7 0.3 57.4 57.4 0.1 Succinic Anhydride 0.0 5.7 5.7 98.9 2243.7 7.1 91.8 2335.5 0.0 2335.5 2.3 2333.2 Nitrogen 2238.0 11187.0 11187.0 11187.0 0.0 11187.0 0.0 0.0 0.0 0.0 0.0 0.0 Oxygen 525.0 965.0 550.5 550.5 0.0 550.5 0.0 0.0 0.0 0.0 0.0 0.0 Butane 100.0 113.4 17.0 16.8 0.2 16.7 0.1 0.3 0.3 0.0 0.0 0.0 Carbon Dioxide 0.0 61.6 77.1 77.1 0.0 77.0 0.0 0.1 0.1 0.0 0.0 0.0 Carbon Monoxide 0.0 61.7 77.1 77.1 0.0 77.1 0.0 0.0 0.0 0.0 0.0 0.0 Water 0.0 284.2 424.1 375.2 48.9 355.2 20.0 68.9 68.6 0.3 0.3 0.0 Total (Kmol/h) 2863.0 12678.7 12396.4 12382.9 2350.4 12270.8 112.1 2462.6 69.2 2393.3 60.1 2333.3 Pressure(Kpa) 200.0 200.0 200.0 150.0 109.0 109.0 109.0 101.0 101.0 101.0 101.0 101.0 Temperature (K) 300.0 350.0 700.0 400.0 395.0 395.0 395.0 395.0 369.0 514.0 475.0 536.6 Vapor Fraction 1.0 1.0 1.0 1.0 0.0 1.0 0.0 0.0 0.0 0.0 0.0 0.0 μp μfs S0 R1 μ92 Maleic Anhydride 57.3 0.1 0.0 0.1 0.0 Succinic Anhydride 0.0 2.3 1.3 5.7 1.4 Nitrogen 0.0 0.0 0.0 8949.6 2237.4 Oxygen 0.0 0.0 0.0 440.4 110.1 Butane 0.0 0.0 0.0 13.4 3.3 Carbon Dioxide 0.0 0.0 0.0 61.6 15.4 Carbon Monoxide 0.0 0.0 0.0 61.7 15.4 Water 0.3 0.0 0.0 284.2 71.0 Total (Kmol/h) 57.7 2.4 0.0 9816.7 2454.2 Pressure(Kpa) 101.0 101.0 101.0 101.0 101.0 Temperature (K) 474.6 532.9 394.0 394.0 394.0 0.0 0.0 0.0 1.0 1.0 Components Vapor Fraction Lo 2337.0 0.0 ENERGY BALANCE The heat contents of all the streams are evaluated and heating and cooling duties for all the heat exchangers in the process determined. Kinetic and potential energies are neglected, and only enthalpy changes for the streams are considered. It is assumed that there is no ΔH of mixing, or pressure effects on ΔH. A standard reference of 298 K and one 1 atm or 101 Kpa of pressure is chosen. The enthalpy of each stream is now considered in turn, using the following enthalpy correlations: To calculate the enthalpies of vapor mixtures the following correlation is used, ΔHv (T, y) = ΔHf + ΔHT = ∑k yk Hf, k (T1) + ∑k yk ∫ Cpo, k (T) dT To calculate the enthalpies of liquid mixtures the following correlation is used, ΔHLk (T) = ΔHof, k + ∫Cpo, k (T) dT - ΔHkvap For the reactor the following expression is used, QR = μ2ΔHv (T, y2) – μ1ΔHv (T, y1) QR is the heat of reaction, which is positive for an endothermic reaction and negative for an exothermic reaction. ΔHv (T, y2) = ΔHout and ΔHv (T, y1) = ΔHin With the stream enthalpies, and stream temperatures, the heating and cooling duties can now be calculated Stream μ0 μ1 μ2 μ31 μ32 μ41 μ42 R1 μ92 Flow (Kmol/h) 2863.0 12678.7 12396.4 12382.9 2350.4 12270.8 112.1 9816.7 2454.2 Pressure (Kpa) 200 200 200 150 150 109 109 200 101 Temperature (K) 300 372 619.3 400 400 393 393 393 393 Enthalpy (KJ/h) -1.24E07 -8.9E07 -4.78E08 -1.45E08 -1.28E09 -9.62E07 -5.56E07 -7.66E07 -1.96E07 Sizing All the process equipments are sized for cost considerations based on the procedure given in the text book. Splitter is sized as a reverse mixer that the output flow is considered for sizing other than the input flow considered for mixers. For the heat exchangers the area of heat transfer and the amount of cooling water required have been calculated. The values of overall heat transfer coefficients are obtained from the book. Since nitrogen is the one carrying the maximum heat, it is considered as the main component from which heat has to be removed. Sizing of compressors is based on the assumption that the expansion is ideal, isentropic and adiabatic giving a Gamma value of 1.4 for an ideal system. Reactor Design is done by assuming a Space velocity or residence time and also that the reactor volume is twice that of the volume occupied by the catalyst. Sizing Reactor: Reacto r Volume (m2) Outside tube diameter (m) Inside tube diameter (m) Inner cross sectional area (m2) Tube Length (m) Inside volume of each tube (m2) No. of tubes Outside surface area of each tube (m2) Multi Tubular 4.492676 0.035 0.02892 0.0006565 6.09 0.003998 1123.625 0.669291 Mixer Fl Τ (residence time) (1/hr) Temp (K) Molar Density of the flow (kmol/m3) Volume (m3) Diamete r (m) Length (m) Area (m2) M1 2863 0,083333 372 188,1388 2.53624 0,931295 3,725181 0,680838 M2 2462.5 0,083333 399 22,13813 12.0502 1,565631 6,262522 1,924191 M3 2337 0,083333 399 15,38897 15.1862 1,691115 6,764462 2,244998 Mixers: Splitter: Splitter Fl Τ (residence time) (1/hr) Temp (K) Molar Density of the flow (kmol/m3) Volume (m3) Diameter (m) Length (m) 12270,8 0,033333 393 184,7362 4,42822 1,1214 4,48566 0,98719 Area (m2) Compressors: ηc ηm (Shaft driven) Compressor P2 P1 T1 γ µ W (kJ/hr) CM1 (For stream R1) 200 109 393 1,4 9816,7 2125869 0,8 0,9 CM2 (For Air Compression) 200 103,2 298 1,4 2763 4981751 0,8 0,9 Wb (shaft driven) (watts) 8,201658291 1,921971726 Heat Exchangers: Heat Exchangers Q U T1 T2 Delta Tln Area(A) (m2) Amount of cooling water(Km ol/hr) HE1 8,38E+07 919,8756 619,3 400 163,6839 556.5563 14800 HE2 1,19E+07 1430,922 536 400 130,1257 63.90984 2100 Delta Tln Condenser: Condenser Q U T1 T2 C 2,64E+06 919,8756 400 394 54,39447 Area(A) (m2) Amount of cooling water (Kmol/hr) 52,76186 467 For the distillation columns, the number of trays and the reflux ratio were determined by the method of Westerberg, assuming ideality. ICAS PDS was used to determine the number of trays for comparative checks. In cases where the method of Westerberg was giving a reflux ratio which when used in ICAS was giving tray number in excess of 100, the method of Underwood was used to determine the minimum reflux ratio and the heuristic of the reflux ratio being 1.2 * the minimum reflux ratio used to get the reflux ratio. An overall column efficiency of 80% is assumed. The column height is calculated using specified values for the tray spacing, extra feed space, disengagement space, skirt height and calculating the height of the tray stack from the number of trays and the value of the tray spacing. The column diameter is calculated by using the Souder Brown equation to determine the maximum allowable vapor velocity based on the column crosssectional area. For the absorber and flash drum, number of theoretical stages calculated by the Kremser equation. Column efficiency is however much lower than distillation columns, generally around 20%, which was the figure used. The column diameter for the absorber is determined where total flows Vj and Lj are largest. This is at the bottom of the column. The diameter is then determined as for distillation column. The solvent recovery unit is a flash drum. The vapor velocity is calculated and is used to determine the column diameter as done for the absorber and the distillation column. Column 1 αlk/hk (avg) N1 25.33 3.54 N2 βlk (ξlk) βhk (1-ξhk) YN NT YR R1 R2 R 3.54 0.995 0.995 0.8 3.54 0.8 0.13 0.13 0.13 Trays Tray Stack Extra Feed Space Diseng Space Skirt Ht Total Ht 7 3.6 1.5 3 1.5 9.6 Bottom of the Column 1 ρl (kg/m3) ρg (Kg/m3) V'(Kg/h) 920.29 2.59 13841.91 L' (Kg/h) σb(dyne/cm ) Flv Csb Uf(ft/s) m/s Db(m) 253198.29 8.76 0.97 0.10 2.13 0.65 1.97 Flv Csb Uf(ft/s) m/s Db(m) 0.01 0.29 4.25 1.04 1.93 Top of the Column 1 ρl (kg/m3) ρg (Kg/m3) V'(Kg/h) L' (Kg/h) σb (dyne/cm) 923.27 0.61 2565.37 1282.68 0.16 Column 2 αlk/hk (avg) N1 4.69 16.29 N2 βlk (ξlk) βhk (1-ξhk) YN NT YR R1 R2 R 16.29 0.999 0.999 0.80 16.29 0.80 0.85 0.85 0.85 Trays Tray Stack Extra Feed Space Diseng Space Skirt Ht Total Ht 50 24.5 1.5 3 1.5 30.5 ρl(kg/m3) ρg (Kg/m3) V'(Kg/h) L' (Kg/h) 568.87 2.27 87064.30 320560.95 Uv (m/s) Dc (m) 0.71555296 4.35772 Column 3 αlk/hk (avg) N1 4.31 11.94 N2 βlk (ξlk) βhk (1-ξhk) YN NT YR R1 R2 R N 17.53 0.99 1.00 0.80 16.41 0.80 0.75 0.94 0.90 20.51 Trays Tray Stack Extra Feed Space Diseng Space Skirt Ht Total Ht 20 9.5 1.5 3 1.5 15.5 ρl (kg/m3) ρg (Kg/m3) V' (Kg/h) L' (Kg/h) 634.78 2.28 11536.25 11769.68 Uv (m/s) 0.75364887 Dc (m) 1.54079 Condensers and Reboilers Overall heat transfer Coefficient (U) (kJ/hr.m2.0K) Area (m2) 97.4 4292,767 2.075829299 373 509 1430,922 14.91611077 373 552 1430,922 16.07479274 Condenser No. Qc (kJ/hr) Tcond (K) Circulating Cooling water Tin (K) Tout (K) Amount (kmol) C1 3,82E+05 372 298 350 C2 2,88E+06 473,89 298 C3 3,12E+06 474,58 298 Reboiler No. QB (kJ/hr) Treb (K) Circulating Steam Tin (K) Tout (K) Amount (kmol) R1 7,73E+07 468,93 1000 488,93 R2 8,73E+07 536,54 1000 R3 2,92E+06 532,92 1000 Overall heat transfer Coefficient (U) (kJ/hr.m2.0K) Area (m2) 1010 4292,767 101.7213 556,54 1170 1430,922 131.6394 552,92 39.2 1430,922 4.368935 Absorber Trays Tray Stack Extra Feed Space 23 11 1.5 Diseng Space Skirt Ht Total Ht 3 1.5 17 ρl (kg/m3) ρg (Kg/m3) V' (Kg/h) L' (Kg/h) Uv (m/s) Dc (m) 1157.01 1.28 351433.04 231038.67 1.36 4.23 Pumps Pump 1 Right Elbows 2.00 Leq Gate Valves Leq Check Leq Z1-Z2 64.00 1.00 7.00 1.00 170.00 4.00 I.D. (m) Ac (m2) Length 1.00 0.79 25.00 Velocity μ Re e e/D R/ρv2 Hf ΔPtotal (m) Wp (KW) 7.06 5.69E-05 1494573.9 4.60E-05 4.60E-05 1.50E-03 75.74 79.74 87.15 Right Elbows Leq Gate Valves Leq Check Leq Z1-Z2 I.D.(m) Ac(m2) Length 2.00 64.00 1.00 7.00 1.00 170.00 25.00 1.00 0.79 25.00 Pump 2 Velocity μ Re e e/D R/ρv2 Hf 8.977745 1.56E-05 5.42E+06 0.000046 4.60E-05 0.001125 79.13129 ΔPtotal (m) Wp (KW) 104.13129 113.2027 Pump 3 Right Elbows Leq 2 Gate Valves 10.47 1 Leq Check 1.15 1 Leq Z1-Z2 27.82 I.D. (m) Ac (m2) Length 0.16 0.021 029 25 6.5 Velocity μ Re e e/D R/ρv2 Hf ΔPtotal (m) Wp (KW) 7.97 5.06E-07 2.51E+07 0.000046 2.81E-04 1.75E-03 162.89 169.39 4.51 Right Elbows Leq Gate Valves Leq Check Leq Z1-Z2 I.D. (m) 2 64.00 1 7.00 0 0.00 0 1.00 Pump 4 Ac (m2) 0.7855 Length 25 Velocity μ Re e e/D R/ρv2 Hf ΔPtotal (m) Wp (KW) 8.79 1.00E-06 8.27E+07 0.000046 4.60E-05 1.25E-03 11.86 11.86 12.58 Pump 5 Right Elbows 0 Leq Gate Valves Leq Check Leq 0.00 1 0.15 0 0.00 Z1-Z2 I.D. (m) Ac(m2) Length 0 0.02 0.00038 25 Velocity μ Re e e/D R/ρv2 Hf ΔPtotal (m) Wp (KW) 10.11 5.90E-05 3.54E+04 0.000046 2.09E-03 2.75E-03 1230.65 1230.65 0.73 Leq Gate Valves Pump 6 Right Elbows 2 64.00 1 Leq 7.00 Check Leq Z1-Z2 I.D. (m) 1 170.0 0 14 1.00 Ac (m2) 0.7855 Length 25 Velocity μ Re e e/D R/ρv2 Hf ΔPtotal (m) Wp (KW) 8.80 5.90E-05 1.40E+06 0.000046 4.60E-05 2.75E-03 176.27 190.27 202.10 Costing and Project Evaluation Distillation Columns, Flash Drum and Absorber Column # Type Height (Ft) Diameter BC($US) UF MF MPF BMC($US) 1 D. C 31.68 3.531 6342.824 3.86261 4.23 1 103634.4 2 D. C 91.5 14.52 66095.23 3.86261 4.23 1 1079919 3 D. C 51.15 5.082 13704.26 3.86261 4.23 1 223911.6 4 Abs 56.1 13.959 42668.86 3.86261 4.23 1 697159.4 5 Abs 56.1 13.959 42668.86 3.86261 4.23 1 697159.4 6 F.D. 56.1 14.025 42880.72 3.86261 4.23 1 700620.9 7 F.D. 56.1 14.025 42880.72 3.86261 4.23 1 700620.9 Stack Ht BC UF MF MPF BMC($US) Total($US) 11.88 485.068 3.862609 1 1.4 2623.079 106257.44 80.85 24214.76 3.862609 1 1.4 130945 1210864.1 31.35 2108.044 3.862609 1 1.4 11399.57 235311.14 36.3 10517.86 3.862609 1 1.4 56876.9 754036.34 36.3 10517.86 3.862609 1 1.4 56876.9 754036.34 Heat Exchangers HX Area(ft2) BC($US) MF MPF UF BMC Reactor 8094.5 35314.62 3.29 2.529 3.862609 657343.2 H1 5930 28848.06 3.29 2.529 3.862609 536975.2 H2 688 7113.175 3.29 0.85 3.862609 86272.79 H3 570 6294.332 3.29 0.85 3.862609 76341.38 C1 26.36 311.4985 1.83 0.85 3.862609 2021.371 C2 63.14 318.0975 1.83 0.85 3.862609 2064.192 C3 83.14 320.2053 1.83 0.85 3.862609 2077.871 R1 1095.00 9621.664 3.29 2.529 3.862609 179096.8 R2 1417.00 11376.83 3.29 2.529 3.862609 R3 47.40 315.9157 1.83 0.85 3.862609 2050.035 211767.4 Total $1,756,010.1 8 Pumps # (Hp) D (inches) 1 117 39 34917.21 7675.11 4.81 0.510 0.05 7.5-250 42592.324 2 150 39 34917.21 9908.91 5.41 0.312 0.10 1-7.5 44826.124 3 6.0434 6 11823.23 911.52 12734.749 4 17 39 34917.21 1319.25 36236.466 5 1 1 4419.90 369.21 4789.1126 6 271 39 34917.21 18687.5 53604.726 Capacity Cost ($US) Motor ($US) a1 a2 a3 HP Total Cost Total $194,783.5 Compressors Number Capacity BC($US) MF MPF UF BMC Total 1 11 4203.395 3.11 1 3.862609 50494.18 $64,082.38 2 2 1131.152 3.11 1 3.862609 13588.2 UF MF MPF BMC($US) Mixers and Splitters Mixer/Splitter Area Height Diameter BC ($US) 1 7.32 12.22 3.055 2518.5 3.8626 4.23 1 41149.506 2 20.71 20.54 5.13 4182.2 3.8626 3.18 1 51370.603 3 24.16 22.19 5.54 4789.6 3.8626 3.18 1 58831.994 4 10.62 14.71 3.67 3548.2 3.8626 4.23 1 57975.006 Total 209327.11 Costing of entire Project Fix Capital Capital Investment Equipment + PI Building and Site Working Capital Fixed and Working Capital 10,864,669.30 4,345,867.72 2,950,844.18 18,161,381.21 Raw Materials Unit Amount Price ($US) Total n-Butane Kmol/h 876000 2.3481692 2,056,996.22 Succinic Anhydride Kmol/h 11563.2 800.592 9,257,405.41 Maintenance % Plant Cost 5 Labour $US/man*yr 15 40000 600,000.00 Manager $US/man*yr 1 200000 200,000.00 Insurance %Plant Cost 2 Lab Analyses $US/man*yr 1 70000 70,000.00 0 0 0 0.017488189 177462.8666 Steam 908,069.06 363,227.62 Cooling Water $US/Kmol/h 10147584 Plant Overheads %Labour Cost 50 300000 Taxes % Fix Capital 2 304210.7405 Revenue $US(Kmol Product/h) 501948 Total Operating Cost 14,237,371.93 44.1261 22,149,007.64 Profit After Tax 7,911,635.72 ROI Pay out Time NPV Rate of Return (NPV = 0) IRR 0.435629627 2.210530747 56,420,932.00 $0.00 0.43562 NPV = 0 2E-07 N i 2.7 0.1 ECONOMIC EVALUATION With all the equipment size and cost, we now proceed to assess the economic viability of the project The capital investment is calculated. The capital Investment which is all the cost incurred at the beginning of the plant life is composed of two components: Fix capital and working capital. The equipment cost plus 25% contingency, represents a part of the fix capital investment. The other component is the cost for building and site, this is generally 40% of the bare module cost The working capital is all the funds require to operate the plant due to delays in payment and maintenance of inventories The other cost to consider is the cost of operating the plant. These costs are continuous over the entire life of the plant. These costs are broken down into the following parts: Raw material costs Cost of utilities Labour Supervision Laboratory analyses Maintenance Plant Overheads/Supplies e.g. Office supplies and spares and sales costs etc. Taxes Insurance The net revenue generated by operating the plant, will be the amount made by selling the product produced, minus all the operating expenses Steam utility and electricity was not included in utility cost, because with heat integration, it was obvious that there are large amounts of heat available for the process that could be used for generating steam and electricity to operate the plant The project was evaluated in terms of the following markers: Net Present Worth An internal rate of return, IRR, also refers to as the minimum attractive rate of return, MARR, was computed The minimum payback period, at NPV = 0 was computed The process is found to be highly profitable The MARR is 43.5%, well above the 10% interest rate used for computing the NPV. Pay back period is computed to be 2.7 years NPV is computed to be highly positive Sensitivity Analyses Sensitivity analyses were done, using the following markers: A sharp increase in raw material cost. A 50% increase in the price of butane was used. The process remained profitable A 50% decrease in product price. The product was no longer profitable. This indicates that the profitability of the process is highly sensitive to sale price of the product. The minimum price the product can be sold for and the process remains profitable is $32.5/Kmol. This represent a 26% decrease in current selling price. High increase in interest rates. If the interest rates exceeds the MARR, then the process no longer remains profitable. Doing the analyses with an interest rate of 50%, the process becomes highly non-profitable, with a highly negative NPV and a pay back period of over a hundred years. Sensitivity analyses 1) A sharp increase in raw material cost. A 50% increase in the price of butane was used. Raw Materials Unit Amount Price ($US) Total n-Butane Kmol/h 876000 3.5222538 3,085,494.33 Succinic Anhydride Kmol/h 11563.2 800.592 9,257,405.41 Maintenance % Plant Cost 5 Labour $US/man*yr 15 40000 600,000.00 Manager $US/man*yr 1 200000 200,000.00 Insurance %Plant Cost 2 Lab Analyses $US/man*yr 1 70000 70,000.00 0 0 0 0.017488189 177462.8666 Steam 908,069.06 363,227.62 Cooling Water $US/Kmol/h 10147584 Plant Overheads %Labour Cost 50 300000 Taxes % Fix Capital 2 304210.7405 Revenue $US (Kmol Product/h) 501948 Total Op. Cost 15,265,870.03 44.1261 22,149,007.64 Profit After Tax 6,883,137.61 ROI Pay out Time NPV NPV = 0(IRR) IRR 0.379 2.526854178 46,725,368.29 ($0.00) 0.37897 NPV = 0 5.59E-08 N i 3.2 0.1 2) A 50% decrease in product cost. Raw Materials Unit Amount Price ($US) Total n-Butane Kmol/h 876000 2.3481692 2,056,996.22 Succinic Anhydride Kmol/h 11563.2 800.592 9,257,405.41 Maintenance % Plant Cost 5 Labour $US/man*yr 15 40000 600,000.00 Manager $US/man*yr 1 200000 200,000.00 Insurance %Plant Cost 2 Lab Analyses $US/man*yr 1 70000 70,000.00 0 0 0 0.017488189 177462.8666 Steam 908,069.06 363,227.62 Cooling Water $US/Kmol/h 10147584 Plant Overheads %Labour Cost 50 300000 Taxes % Fix Capital 2 304210.7405 Revenue $US (Kmol Product/h) 501948 Total Op. Cost 14,237,371.93 22.06305 11,074,503.82 Profit After Tax -3,162,868.10 3) High increases in interest rates Raw Materials Unit Amount Price ($US) Total n-Butane Kmol/h 876000 2.3481692 2,056,996.22 Succinic Anhydride Kmol/h 11563.2 800.592 9,257,405.41 Maintenance % Plant Cost 5 Labour $US/man*yr 15 40000 600,000.00 Manager $US/man*yr 1 200000 200,000.00 Insurance %Plant Cost 2 Lab Analyses $US/man*yr 1 70000 70,000.00 0 0 0 0.017488189 177462.8666 Steam 908,069.06 363,227.62 Cooling Water $US/Kmol/h 10147584 Plant Overheads %Labour Cost 50 300000 Taxes % Fix Capital 2 304210.7405 Revenue $US (Kmol Product/h) 501948 Total Op Cost 14,237,371.93 44.1261 22,149,007.64 Profit After Tax 7,911,635.72 NPV -2,338,192.29 Heat Integration Tin (K) Flow Tout (K) Enthalpy in (kJ) Enthalpy out (kJ) Available Heat No. Streams Condition 1 u2 Hot 12396.4 619.3 400 9.84E+06 3.07E+06 -6.77E+06 2 Lo Hot 2336.97 536 400 -5.05E+08 -5.56E+08 -5.10E+07 3 He1cooling water Cold 14800 298 373 0 1.59E+07 1.59E+07 4 He2cooling water Cold 2100 298 373 0 1.59E+07 1.59E+07 5 C1 Cold 67.6 298 350 0 1.19E+07 1.19E+07 6 C2 Cold 509 298 373 0 1.59E+07 1.59E+07 7 C3 Cold 552 298 373 0 1.59E+07 1.59E+07 8 R1 Hot 15900 1000 488.93 8.10E+07 6.34E+07 -1.76E+07 9 R2 Hot 18000 1000 556.54 8.10E+07 6.57E+07 -1.53E+07 10 R3 Hot 602 1000 552.92 8.10E+07 6.55E+07 -1.54E+07 11 u31 Hot 12396.4 400 394 3.83E+07 3.56E+07 -2.70E+06 12 Ccooling water Cold 467 298 373 0 1.59E+07 1.59E+07 The PA tool box of ICAS was used to generate the Pinch Diagrams after giving all the streams input data. The Diagrams shows an additional cooling of 1.0811E11 kJ/hr. So this is the amount of excess heat which can be used for other purposes. The pinch point is at 394K for the hot stream and 383K for the cold stream obtained from the cascade diagram. The results shows an additional of 3 heat exchangers are needed to satisfy the condition. The heat duties have been added up and found that the process has excess heat than required in the process. This can be attributed to the highly exothermic reactions in the reactor. Environmental Impact Analysis Streams In Streams Out µ01 µ92 S0 µ61 µp Total PEI HTPI HTPE ATP TTP GWP ODP PCOP AP Input Sum 8740.31 1696.89 0.90263 448.692 1696.89 0 0 4896.93 0 Output Sum 12064.7 5060.28 1668.93 98.6746 5060.28 0.21655 0 176.289 0 Impact Generated 3324.36 3363.39 1668.02 -350.017 3363.39 0.21655 0 -4720.64 0 Analysis: The Report generated gives a higher value of the Total Potential Environmental Impact suggesting that the process has to be modified for environmental purposes. The high value of the PEI is because of the excess amounts of carbon dioxide released into the atmosphere. By analyzing all the individual output streams, it can be clearly observed that output stream 3 has quiet high values of the total PEI. It is because of the release of the purge gas from the splitter directly into the atmosphere. As a process improvement step, we can use incinerator to convert the Carbon monoxide to carbon dioxide before it is released into the atmosphere. As an alternative a scrubber can be used to scrub all the harmful gases and prevent them from entering into the atmosphere. The other 2 outlet streams mostly contain water other than the product, so they have less environmental impact. Changing the solvent in the absorption column from Succinic anhydride to water can increase the environmental attractiveness of the process but the required product yield cannot be attained.