Homework2
advertisement

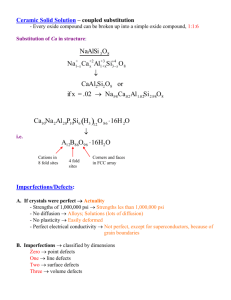
Gina Cavallo MANE 6940 June 3, 2014 1. Explain briefly (~ 200 words each) basic facts about the atomic, microscopic and macroscopic structures of the following materials: Metals and Alloys Ceramics Polymeric Materials Composites Metals and Alloys: The atoms of metals and alloys are held together by forces between electrons of adjacent atoms. The atom radius size varies from 10^-7 to 10^-6 mm. Metals vary in phases depending on the temperature, the most common phases being the α and ϒ phases. They are mostly created in a liquid state and form their structure upon solidifying when cooled. As the temperature of the metal/alloy decreases, the distances between atoms decrease. Then, the distance at which bonding occurs is reached at random sites, which produces the first nuclei of crystal growth. Metals and alloys typically have simple cubic, body centered cubic, face centered cubic, and hexagonal close packed crystal structures. They can also have defects in the crystal lattice, such as point, line, and surface defects. The 3 main types of point defects are vacancies, interstitial atoms, and substitutional atoms. Point defects can strengthen an alloy. Surface defects are the separation between crystals or parts of a crystal. Line defects are defects in the arrangement of atoms in two perpendicular directions and of macroscopic size in the third direction. Class Textbook: Mechanics of Solid Materials, J. Lemaitre, J.-L. Chaboche Ceramics Most ceramics are compounds, made up of 2 or more elements. The most common atomic bonds are covalent and ionic bonds, which are stronger than metallic bonding. Therefore, ceramics tend to have high hardness and high compressive strength. It’s also why they’re less ductile and have lower tensile strength. There are no free electrons, therefore, ceramics do not easily conduct electricity and heat. The crystal structures of ceramics differ, resulting in a varying range of properties. For example, some have high temperatures superconductivity. The microstructure can vary from glassy, completely crystalline, or a combination of the two. Silicate ceramics are the basic structure for many ceramics. It has a pyramid atomic arrangement. Ceramic crystals have imperfections such as point defects. The defect formation is mainly based on the condition of charge neutrality of the atoms. One type of defect is the Frenkel-defect, which happens when a host atom moves to a close interstitial location to create a vacancyinterstitial pair of cations. A Schottky-defect is a pair of nearby cation and anion vacancies. http://www.ndted.org/EducationResources/CommunityCollege/Materials/Structure/ceramic.htm Class Textbook: Mechanics of Solid Materials, J. Lemaitre, J.-L. Chaboche Poylmeric Materials Polymers are often transparent and comprise of carbon atoms in a chain arrangement. They mainly consist of covalent bonds between carbon atoms. Hydrogen, Oxygen, and Nitrogen atoms can also be found attached to the chain. These chains of molecules can be structured randomly in amorphous polymers, but have some order on a smaller scale. The chains can also become arranged in packs, forming crystallites, which form a superstructure. Depending on the temperature, they are found in 4 different states, glass state, transition state, rubbery state, and fluid state. Polymers are flexible, but their chains don’t have a uniform structure on a molecular scale. Class Textbook: Mechanics of Solid Materials, J. Lemaitre, J.-L. Chaboche Composites Composites are made from 2 or more materials combined to produce a material with a different characteristic than each of the individual parts. They typically have a bulk phase, the matrix, and a non-continous phase, the reinforcement. The bulk phase spreads the load over a large surface area, and transfers it to the reinforcement material, which can withstand a larger load. Reinforcements usually hinder crack propagation. The fibres in fibre-resin composites are arranged in either unidirectional (fibers are parallel), bidirectional (by crossing of unidirectional sheets), and tridirectional. They are anisotropic (directionally dependent). http://www.ndted.org/EducationResources/CommunityCollege/Materials/Structure/composite.htm 2.- Explain briefly (~ 100 words each) basic facts about the microscopic processes that take place when the following materials are subjected to gradually increasing mechanical loads: Metals and Alloys Ceramics Polymeric Materials Composites Metals and Alloys Under an external load, a slip displacement may occur, in which an edge or screw dislocation moves across a crystal and irreversible displacement occurs. Bonds break only in the location of the dislocation line. An edge dislocation can also move perpendicular to its slip plane, creating a climb displacement. During uniaxial external loading, which first increases and then decreases, the polycrystal deforms in the following sequence: First, it experiences elastic deformation. Then, the elastic limit is the point at which the state of stress or strain causes the first irreversible movements of dislocations. Then plastic deformation occurs. The phenomenon of hardening can occur after this, then, viscoplastic deformation, and finally restoration or recovery. Fracture can also occur, which destroys the cohesion of the matter by creating surface or volume discontinuities within the material. Brittle fractures involves only the fracture of interatomic bonds. Lattice defects or geometrical imperfections result in stress concentration, and begin the fracture process. Ductile fracture occurs when large local deformations occur at the location of crystalline defects. This instability causes decohesion at the interface or a fracture, creating a microcrack or a cavity. Under a cyclic load, fatigue failure starts with nucleation. Cyclic plastic microdeformations occur near defects, and further slip is prevented due to the increase of dislocation nodes. This is followed by the initiation of microcracks, the growth of microcracks, and the growth of a macrocack. Class Textbook: Mechanics of Solid Materials, J. Lemaitre, J.-L. Chaboche Ceramics Ceramics are brittle. When they are under tension, preexisting cracks amplify the stress and create a single main crack at the top of a flaw. This crack will quickly propagate and cause the material to break. However, under compression, the mechanical properties of ceramics are fairly good. By nature, ceramics contain cracks and defects. The higher number of these defects a ceramic material has, the lower its modulus of elasticity. http://www.sv.vt.edu/classes/MSE2094_NoteBook/97ClassProj/exper/gordon/www/c eramic.html Polymers Polymer deformations are generally elastic when the mechanical loads are below a certain value. There is a relative movement of chain segments which is thermally activated, but the bonds are not destroyed. Above a certain load, there is irreversible reorientation of chain segments resulting in additional strains beside the elastic stains. These strains remain for a while after the load is removed. A high load destroys the substructure of the crystallite by breaking the weakest link while new bonds develop. The combination of external loads and thermal activation breaks molecular bonds, resulting in fractures. The initiation zones are usually those with defects, impurities, or crystalline flaws. Composites Composites are resistant to fatigue damage, and are repairable. However, repeated cyclic loads can cause the laminates to separate between layers. Individual fibers can break out from the matrix. Failures in compression can occur at the individual reinforcing fibers in compression. Failures in tension can occur when one or multiple layers fail in tension of the matrix or when the bonds between the matrix and the fibers is broken. http://en.wikipedia.org/wiki/Composite_material http://www.ndted.org/EducationResources/CommunityCollege/Materials/Structure/composite.htm Other Sources Class Textbook: Mechanics of Solid Materials, J. Lemaitre, J.-L. Chaboche